EMD-11276
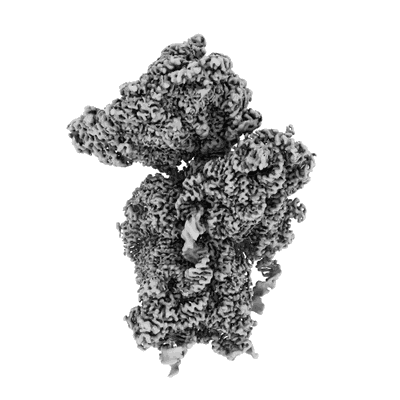
SARS-CoV-2 Nsp1 bound to the human 40S ribosomal subunit
EMD-11276
Single-particle2.6 Å
 Deposition: 01/07/2020
Deposition: 01/07/2020Map released: 29/07/2020
Last modified: 01/05/2024
Sample Organism:
Homo sapiens,
Severe acute respiratory syndrome coronavirus 2
Sample: SARS-CoV-2 Nsp1 bound to the human 40S ribosomal subunit
Fitted models: 6zlw (Avg. Q-score: 0.595)
Deposition Authors: Thoms M ,
Buschauer R
,
Buschauer R 
Sample: SARS-CoV-2 Nsp1 bound to the human 40S ribosomal subunit
Fitted models: 6zlw (Avg. Q-score: 0.595)
Deposition Authors: Thoms M
 ,
Buschauer R
,
Buschauer R 
Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2.
Thoms M  ,
Buschauer R
,
Buschauer R  ,
Ameismeier M
,
Ameismeier M  ,
Koepke L
,
Koepke L  ,
Denk T
,
Denk T  ,
Hirschenberger M
,
Hirschenberger M  ,
Kratzat H
,
Kratzat H  ,
Hayn M,
Mackens-Kiani T
,
Hayn M,
Mackens-Kiani T  ,
Cheng J
,
Cheng J  ,
Straub JH,
Sturzel CM,
Frohlich T
,
Straub JH,
Sturzel CM,
Frohlich T  ,
Berninghausen O
,
Berninghausen O  ,
Becker T
,
Becker T  ,
Kirchhoff F
,
Kirchhoff F  ,
Sparrer KMJ
,
Sparrer KMJ  ,
Beckmann R
,
Beckmann R 
(2020) Science , 369 , 1249 - 1255
 ,
Buschauer R
,
Buschauer R  ,
Ameismeier M
,
Ameismeier M  ,
Koepke L
,
Koepke L  ,
Denk T
,
Denk T  ,
Hirschenberger M
,
Hirschenberger M  ,
Kratzat H
,
Kratzat H  ,
Hayn M,
Mackens-Kiani T
,
Hayn M,
Mackens-Kiani T  ,
Cheng J
,
Cheng J  ,
Straub JH,
Sturzel CM,
Frohlich T
,
Straub JH,
Sturzel CM,
Frohlich T  ,
Berninghausen O
,
Berninghausen O  ,
Becker T
,
Becker T  ,
Kirchhoff F
,
Kirchhoff F  ,
Sparrer KMJ
,
Sparrer KMJ  ,
Beckmann R
,
Beckmann R 
(2020) Science , 369 , 1249 - 1255
Abstract:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the current coronavirus disease 2019 (COVID-19) pandemic. A major virulence factor of SARS-CoVs is the nonstructural protein 1 (Nsp1), which suppresses host gene expression by ribosome association. Here, we show that Nsp1 from SARS-CoV-2 binds to the 40S ribosomal subunit, resulting in shutdown of messenger RNA (mRNA) translation both in vitro and in cells. Structural analysis by cryo-electron microscopy of in vitro-reconstituted Nsp1-40S and various native Nsp1-40S and -80S complexes revealed that the Nsp1 C terminus binds to and obstructs the mRNA entry tunnel. Thereby, Nsp1 effectively blocks retinoic acid-inducible gene I-dependent innate immune responses that would otherwise facilitate clearance of the infection. Thus, the structural characterization of the inhibitory mechanism of Nsp1 may aid structure-based drug design against SARS-CoV-2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the current coronavirus disease 2019 (COVID-19) pandemic. A major virulence factor of SARS-CoVs is the nonstructural protein 1 (Nsp1), which suppresses host gene expression by ribosome association. Here, we show that Nsp1 from SARS-CoV-2 binds to the 40S ribosomal subunit, resulting in shutdown of messenger RNA (mRNA) translation both in vitro and in cells. Structural analysis by cryo-electron microscopy of in vitro-reconstituted Nsp1-40S and various native Nsp1-40S and -80S complexes revealed that the Nsp1 C terminus binds to and obstructs the mRNA entry tunnel. Thereby, Nsp1 effectively blocks retinoic acid-inducible gene I-dependent innate immune responses that would otherwise facilitate clearance of the infection. Thus, the structural characterization of the inhibitory mechanism of Nsp1 may aid structure-based drug design against SARS-CoV-2.
