EMD-12910
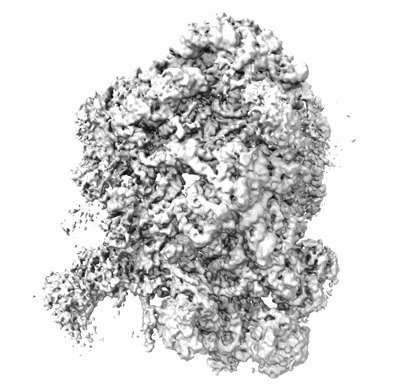
Nog1-TAP associated immature ribosomal particles from S. cerevisiae after rpL2 expression shut down, population C
EMD-12910
Single-particle3.9 Å
 Deposition: 11/05/2021
Deposition: 11/05/2021Map released: 03/11/2021
Last modified: 10/07/2024
Sample Organism:
Saccharomyces cerevisiae (strain ATCC 204508 / S288c)
Sample: Nog1-TAP associated immature ribosomal particles from cells depleted of rpL2.
Fitted models: 7ohv (Avg. Q-score: 0.324)
Raw data: EMPIAR-10780
Deposition Authors: Milkereit P ,
Poell G
,
Poell G
Sample: Nog1-TAP associated immature ribosomal particles from cells depleted of rpL2.
Fitted models: 7ohv (Avg. Q-score: 0.324)
Raw data: EMPIAR-10780
Deposition Authors: Milkereit P
 ,
Poell G
,
Poell G
Analysis of subunit folding contribution of three yeast large ribosomal subunit proteins required for stabilisation and processing of intermediate nuclear rRNA precursors.
Poll G,
Pilsl M,
Griesenbeck J  ,
Tschochner H,
Milkereit P
,
Tschochner H,
Milkereit P 
(2021) PLoS One , 16 , e0252497 - e0252497
 ,
Tschochner H,
Milkereit P
,
Tschochner H,
Milkereit P 
(2021) PLoS One , 16 , e0252497 - e0252497
Abstract:
In yeast and human cells many of the ribosomal proteins (r-proteins) are required for the stabilisation and productive processing of rRNA precursors. Functional coupling of r-protein assembly with the stabilisation and maturation of subunit precursors potentially promotes the production of ribosomes with defined composition. To further decipher mechanisms of such an intrinsic quality control pathway we analysed here the contribution of three yeast large ribosomal subunit r-proteins rpL2 (uL2), rpL25 (uL23) and rpL34 (eL34) for intermediate nuclear subunit folding steps. Structure models obtained from single particle cryo-electron microscopy analyses provided evidence for specific and hierarchic effects on the stable positioning and remodelling of large ribosomal subunit domains. Based on these structural and previous biochemical data we discuss possible mechanisms of r-protein dependent hierarchic domain arrangement and the resulting impact on the stability of misassembled subunits.
In yeast and human cells many of the ribosomal proteins (r-proteins) are required for the stabilisation and productive processing of rRNA precursors. Functional coupling of r-protein assembly with the stabilisation and maturation of subunit precursors potentially promotes the production of ribosomes with defined composition. To further decipher mechanisms of such an intrinsic quality control pathway we analysed here the contribution of three yeast large ribosomal subunit r-proteins rpL2 (uL2), rpL25 (uL23) and rpL34 (eL34) for intermediate nuclear subunit folding steps. Structure models obtained from single particle cryo-electron microscopy analyses provided evidence for specific and hierarchic effects on the stable positioning and remodelling of large ribosomal subunit domains. Based on these structural and previous biochemical data we discuss possible mechanisms of r-protein dependent hierarchic domain arrangement and the resulting impact on the stability of misassembled subunits.
