EMD-13020
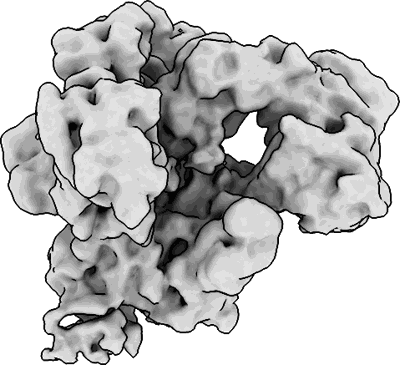
CryoEM structure of DNA Polymerase alpha - primase bound to SARS CoV nsp1
EMD-13020
Single-particle4.12 Å
 Deposition: 01/06/2021
Deposition: 01/06/2021Map released: 10/11/2021
Last modified: 17/07/2024
Sample Organism:
Homo sapiens,
Severe acute respiratory syndrome coronavirus
Sample: Complex of DNA polymerase alpha - primase bound to SARS COV-2 nsp1
Fitted models: 7opl (Avg. Q-score: 0.275)
Raw data: EMPIAR-11477
Deposition Authors: Kilkenny TJ, Pellegrini L
Sample: Complex of DNA polymerase alpha - primase bound to SARS COV-2 nsp1
Fitted models: 7opl (Avg. Q-score: 0.275)
Raw data: EMPIAR-11477
Deposition Authors: Kilkenny TJ, Pellegrini L

Structural basis for the interaction of SARS-CoV-2 virulence factor nsp1 with DNA polymerase alpha-primase.
Kilkenny ML,
Veale CE,
Guppy A,
Hardwick SW,
Chirgadze DY,
Rzechorzek NJ,
Maman JD,
Pellegrini L 
(2022) Protein Sci , 31 , 333 - 344

(2022) Protein Sci , 31 , 333 - 344
Abstract:
The molecular mechanisms that drive the infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-the causative agent of coronavirus disease 2019 (COVID-19)-are under intense current scrutiny to understand how the virus operates and to uncover ways in which the disease can be prevented or alleviated. Recent proteomic screens of the interactions between viral and host proteins have identified the human proteins targeted by SARS-CoV-2. The DNA polymerase α (Pol α)-primase complex or primosome-responsible for initiating DNA synthesis during genomic duplication-was identified as a target of nonstructural protein 1 (nsp1), a major virulence factor in the SARS-CoV-2 infection. Here, we validate the published reports of the interaction of nsp1 with the primosome by demonstrating direct binding with purified recombinant components and providing a biochemical characterization of their interaction. Furthermore, we provide a structural basis for the interaction by elucidating the cryo-electron microscopy structure of nsp1 bound to the primosome. Our findings provide biochemical evidence for the reported targeting of Pol α by the virulence factor nsp1 and suggest that SARS-CoV-2 interferes with Pol α's putative role in the immune response during the viral infection.
The molecular mechanisms that drive the infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-the causative agent of coronavirus disease 2019 (COVID-19)-are under intense current scrutiny to understand how the virus operates and to uncover ways in which the disease can be prevented or alleviated. Recent proteomic screens of the interactions between viral and host proteins have identified the human proteins targeted by SARS-CoV-2. The DNA polymerase α (Pol α)-primase complex or primosome-responsible for initiating DNA synthesis during genomic duplication-was identified as a target of nonstructural protein 1 (nsp1), a major virulence factor in the SARS-CoV-2 infection. Here, we validate the published reports of the interaction of nsp1 with the primosome by demonstrating direct binding with purified recombinant components and providing a biochemical characterization of their interaction. Furthermore, we provide a structural basis for the interaction by elucidating the cryo-electron microscopy structure of nsp1 bound to the primosome. Our findings provide biochemical evidence for the reported targeting of Pol α by the virulence factor nsp1 and suggest that SARS-CoV-2 interferes with Pol α's putative role in the immune response during the viral infection.
