EMD-18389
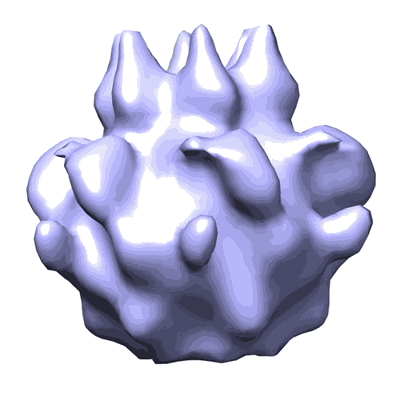
P.aeruginosa clone C construct PaFtsH2-H1-link32 in negative stain
EMD-18389
Single-particle22.8 Å
 Deposition: 05/09/2023
Deposition: 05/09/2023Map released: 17/01/2024
Last modified: 14/02/2024
Sample Organism:
Pseudomonas aeruginosa SG17M
Sample: Construct PFtsH2-H1-link32
Deposition Authors: Mawla GD, Mansour Kamal S, Cao L-Y, Purhonen P, Hebert H, Sauer RT, Baker TA, Romling U
Sample: Construct PFtsH2-H1-link32
Deposition Authors: Mawla GD, Mansour Kamal S, Cao L-Y, Purhonen P, Hebert H, Sauer RT, Baker TA, Romling U
The membrane-cytoplasmic linker defines activity of FtsH proteases in Pseudomonas aeruginosa clone C.
Mawla GD,
Kamal SM,
Cao LY,
Purhonen P,
Hebert H,
Sauer RT,
Baker TA,
Romling U
(2024) J Biol Chem , 300 , 105622 - 105622
(2024) J Biol Chem , 300 , 105622 - 105622
Abstract:
Pandemic Pseudomonas aeruginosa clone C strains encode two inner-membrane associated ATP-dependent FtsH proteases. PaftsH1 is located on the core genome and supports cell growth and intrinsic antibiotic resistance, whereas PaftsH2, a xenolog acquired through horizontal gene transfer from a distantly related species, is unable to functionally replace PaftsH1. We show that purified PaFtsH2 degrades fewer substrates than PaFtsH1. Replacing the 31-amino acid-extended linker region of PaFtsH2 spanning from the C-terminal end of the transmembrane helix-2 to the first seven highly divergent residues of the cytosolic AAA+ ATPase module with the corresponding region of PaFtsH1 improves hybrid-enzyme substrate processing in vitro and enables PaFtsH2 to substitute for PaFtsH1 in vivo. Electron microscopy indicates that the identity of this linker sequence influences FtsH flexibility. We find membrane-cytoplasmic (MC) linker regions of PaFtsH1 characteristically glycine-rich compared to those from FtsH2. Consequently, introducing three glycines into the membrane-proximal end of PaFtsH2's MC linker is sufficient to elevate its activity in vitro and in vivo. Our findings establish that the efficiency of substrate processing by the two PaFtsH isoforms depends on MC linker identity and suggest that greater linker flexibility and/or length allows FtsH to degrade a wider spectrum of substrates. As PaFtsH2 homologs occur across bacterial phyla, we hypothesize that FtsH2 is a latent enzyme but may recognize specific substrates or is activated in specific contexts or biological niches. The identity of such linkers might thus play a more determinative role in the functionality of and physiological impact by FtsH proteases than previously thought.
Pandemic Pseudomonas aeruginosa clone C strains encode two inner-membrane associated ATP-dependent FtsH proteases. PaftsH1 is located on the core genome and supports cell growth and intrinsic antibiotic resistance, whereas PaftsH2, a xenolog acquired through horizontal gene transfer from a distantly related species, is unable to functionally replace PaftsH1. We show that purified PaFtsH2 degrades fewer substrates than PaFtsH1. Replacing the 31-amino acid-extended linker region of PaFtsH2 spanning from the C-terminal end of the transmembrane helix-2 to the first seven highly divergent residues of the cytosolic AAA+ ATPase module with the corresponding region of PaFtsH1 improves hybrid-enzyme substrate processing in vitro and enables PaFtsH2 to substitute for PaFtsH1 in vivo. Electron microscopy indicates that the identity of this linker sequence influences FtsH flexibility. We find membrane-cytoplasmic (MC) linker regions of PaFtsH1 characteristically glycine-rich compared to those from FtsH2. Consequently, introducing three glycines into the membrane-proximal end of PaFtsH2's MC linker is sufficient to elevate its activity in vitro and in vivo. Our findings establish that the efficiency of substrate processing by the two PaFtsH isoforms depends on MC linker identity and suggest that greater linker flexibility and/or length allows FtsH to degrade a wider spectrum of substrates. As PaFtsH2 homologs occur across bacterial phyla, we hypothesize that FtsH2 is a latent enzyme but may recognize specific substrates or is activated in specific contexts or biological niches. The identity of such linkers might thus play a more determinative role in the functionality of and physiological impact by FtsH proteases than previously thought.
