EMD-20084
The Central Role of the Tail in Switching Off Myosin II in Cells
EMD-20084
Single-particle25.0 Å
 Deposition: 10/04/2019
Deposition: 10/04/2019Map released: 24/04/2019
Last modified: 06/09/2023
Sample Organism:
Meleagris gallopavo
Sample: Myosin II
Deposition Authors: Craig R ,
Yang SX,
Lee KH
,
Yang SX,
Lee KH  ,
Woodhead JL
,
Woodhead JL  ,
Sato O
,
Sato O  ,
Ikebe M
,
Ikebe M
Sample: Myosin II
Deposition Authors: Craig R
 ,
Yang SX,
Lee KH
,
Yang SX,
Lee KH  ,
Woodhead JL
,
Woodhead JL  ,
Sato O
,
Sato O  ,
Ikebe M
,
Ikebe M
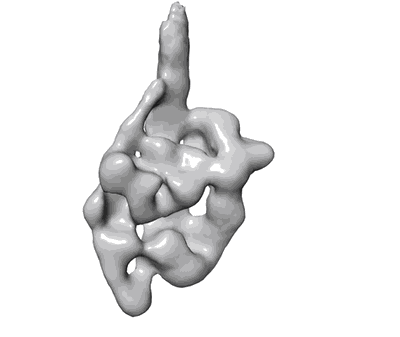
The central role of the tail in switching off 10S myosin II activity.
Abstract:
Myosin II is a motor protein with two heads and an extended tail that plays an essential role in cell motility. Its active form is a polymer (myosin filament) that pulls on actin to generate motion. Its inactive form is a monomer with a compact structure (10S sedimentation coefficient), in which the tail is folded and the two heads interact with each other, inhibiting activity. This conformation is thought to function in cells as an energy-conserving form of the molecule suitable for storage as well as transport to sites of filament assembly. The mechanism of inhibition of the compact molecule is not fully understood. We have performed a 3-D reconstruction of negatively stained 10S myosin from smooth muscle in the inhibited state using single-particle analysis. The reconstruction reveals multiple interactions between the tail and the two heads that appear to trap ATP hydrolysis products, block actin binding, hinder head phosphorylation, and prevent filament formation. Blocking these essential features of myosin function could explain the high degree of inhibition of the folded form of myosin thought to underlie its energy-conserving function in cells. The reconstruction also suggests a mechanism for unfolding when myosin is activated by phosphorylation.
Myosin II is a motor protein with two heads and an extended tail that plays an essential role in cell motility. Its active form is a polymer (myosin filament) that pulls on actin to generate motion. Its inactive form is a monomer with a compact structure (10S sedimentation coefficient), in which the tail is folded and the two heads interact with each other, inhibiting activity. This conformation is thought to function in cells as an energy-conserving form of the molecule suitable for storage as well as transport to sites of filament assembly. The mechanism of inhibition of the compact molecule is not fully understood. We have performed a 3-D reconstruction of negatively stained 10S myosin from smooth muscle in the inhibited state using single-particle analysis. The reconstruction reveals multiple interactions between the tail and the two heads that appear to trap ATP hydrolysis products, block actin binding, hinder head phosphorylation, and prevent filament formation. Blocking these essential features of myosin function could explain the high degree of inhibition of the folded form of myosin thought to underlie its energy-conserving function in cells. The reconstruction also suggests a mechanism for unfolding when myosin is activated by phosphorylation.
