EMD-2206
Negative stained electron microscopy reconstruction of the loop of influenza A virus ribonucleoprotein isolated from virions.
EMD-2206
Single-particle27.0 Å
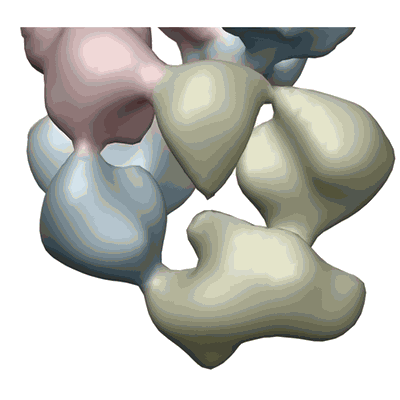 Deposition: 19/09/2012
Deposition: 19/09/2012Map released: 05/12/2012
Last modified: 09/01/2013
Sample Organism:
Influenza A virus
Sample: Native influenza A virus ribonucleoprotein (STRAIN A/WSN/33, H1N1)
Deposition Authors: Arranz R ,
Coloma R
,
Coloma R  ,
Chichon FJ
,
Chichon FJ  ,
Conesa JJ,
Carrascosa JL,
Valpuesta JM
,
Conesa JJ,
Carrascosa JL,
Valpuesta JM  ,
Ortin J
,
Ortin J  ,
Martin-Benito J
,
Martin-Benito J 
Sample: Native influenza A virus ribonucleoprotein (STRAIN A/WSN/33, H1N1)
Deposition Authors: Arranz R
 ,
Coloma R
,
Coloma R  ,
Chichon FJ
,
Chichon FJ  ,
Conesa JJ,
Carrascosa JL,
Valpuesta JM
,
Conesa JJ,
Carrascosa JL,
Valpuesta JM  ,
Ortin J
,
Ortin J  ,
Martin-Benito J
,
Martin-Benito J 
The structure of native influenza virion ribonucleoproteins.
Arranz R  ,
Coloma R
,
Coloma R  ,
Chichon FJ
,
Chichon FJ  ,
Conesa JJ,
Carrascosa JL,
Valpuesta JM
,
Conesa JJ,
Carrascosa JL,
Valpuesta JM  ,
Ortin J
,
Ortin J  ,
Martin-Benito J
,
Martin-Benito J 
(2012) Science , 338 , 1634 - 1637
 ,
Coloma R
,
Coloma R  ,
Chichon FJ
,
Chichon FJ  ,
Conesa JJ,
Carrascosa JL,
Valpuesta JM
,
Conesa JJ,
Carrascosa JL,
Valpuesta JM  ,
Ortin J
,
Ortin J  ,
Martin-Benito J
,
Martin-Benito J 
(2012) Science , 338 , 1634 - 1637
Abstract:
The influenza viruses cause annual epidemics of respiratory disease and occasional pandemics, which constitute a major public-health issue. The segmented negative-stranded RNAs are associated with the polymerase complex and nucleoprotein (NP), forming ribonucleoproteins (RNPs), which are responsible for virus transcription and replication. We describe the structure of native RNPs derived from virions. They show a double-helical conformation in which two NP strands of opposite polarity are associated with each other along the helix. Both strands are connected by a short loop at one end of the particle and interact with the polymerase complex at the other end. This structure will be relevant for unraveling the mechanisms of nuclear import of parental virus RNPs, their transcription and replication, and the encapsidation of progeny RNPs into virions.
The influenza viruses cause annual epidemics of respiratory disease and occasional pandemics, which constitute a major public-health issue. The segmented negative-stranded RNAs are associated with the polymerase complex and nucleoprotein (NP), forming ribonucleoproteins (RNPs), which are responsible for virus transcription and replication. We describe the structure of native RNPs derived from virions. They show a double-helical conformation in which two NP strands of opposite polarity are associated with each other along the helix. Both strands are connected by a short loop at one end of the particle and interact with the polymerase complex at the other end. This structure will be relevant for unraveling the mechanisms of nuclear import of parental virus RNPs, their transcription and replication, and the encapsidation of progeny RNPs into virions.
