EMD-22843
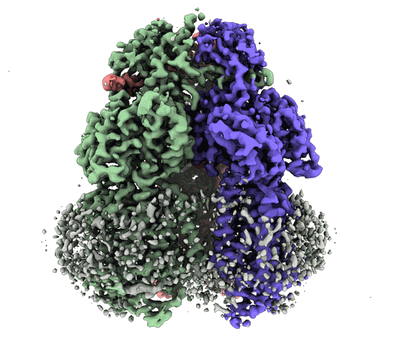
Cryo-electron microscopy structure of the heavy metal efflux pump CusA in the symmetric closed state
EMD-22843
Single-particle3.2 Å
 Deposition: 13/10/2020
Deposition: 13/10/2020Map released: 14/04/2021
Last modified: 06/03/2024
Sample Organism:
Escherichia coli
Sample: CusA in symmetric closed extrusion form
Fitted models: 7kf5 (Avg. Q-score: 0.424)
Deposition Authors: Moseng MA
Sample: CusA in symmetric closed extrusion form
Fitted models: 7kf5 (Avg. Q-score: 0.424)
Deposition Authors: Moseng MA
Cryo-EM Structures of CusA Reveal a Mechanism of Metal-Ion Export.
Moseng MA,
Lyu M,
Pipatpolkai T  ,
Glaza P
,
Glaza P  ,
Emerson CC,
Stewart PL
,
Emerson CC,
Stewart PL  ,
Stansfeld PJ
,
Stansfeld PJ  ,
Yu EW
,
Yu EW 
(2021) Mbio , 12
 ,
Glaza P
,
Glaza P  ,
Emerson CC,
Stewart PL
,
Emerson CC,
Stewart PL  ,
Stansfeld PJ
,
Stansfeld PJ  ,
Yu EW
,
Yu EW 
(2021) Mbio , 12
Abstract:
Gram-negative bacteria utilize the resistance-nodulation-cell division (RND) superfamily of efflux pumps to expel a variety of toxic compounds from the cell. The Escherichia coli CusA membrane protein, which recognizes and extrudes biocidal Cu(I) and Ag(I) ions, belongs to the heavy-metal efflux (HME) subfamily of RND efflux pumps. We here report four structures of the trimeric CusA heavy-metal efflux pump in the presence of Cu(I) using single-particle cryo-electron microscopy (cryo-EM). We discover that different CusA protomers within the trimer are able to bind Cu(I) ions simultaneously. Our structural data combined with molecular dynamics (MD) simulations allow us to propose a mechanism for ion transport where each CusA protomer functions independently within the trimer.IMPORTANCE The bacterial RND superfamily of efflux pumps mediate resistance to a variety of biocides, including Cu(I) and Ag(I) ions. Here we report four cryo-EM structures of the trimeric CusA pump in the presence of Cu(I). Combined with MD simulations, our data indicate that each CusA protomer within the trimer recognizes and extrudes Cu(I) independently.
Gram-negative bacteria utilize the resistance-nodulation-cell division (RND) superfamily of efflux pumps to expel a variety of toxic compounds from the cell. The Escherichia coli CusA membrane protein, which recognizes and extrudes biocidal Cu(I) and Ag(I) ions, belongs to the heavy-metal efflux (HME) subfamily of RND efflux pumps. We here report four structures of the trimeric CusA heavy-metal efflux pump in the presence of Cu(I) using single-particle cryo-electron microscopy (cryo-EM). We discover that different CusA protomers within the trimer are able to bind Cu(I) ions simultaneously. Our structural data combined with molecular dynamics (MD) simulations allow us to propose a mechanism for ion transport where each CusA protomer functions independently within the trimer.IMPORTANCE The bacterial RND superfamily of efflux pumps mediate resistance to a variety of biocides, including Cu(I) and Ag(I) ions. Here we report four cryo-EM structures of the trimeric CusA pump in the presence of Cu(I). Combined with MD simulations, our data indicate that each CusA protomer within the trimer recognizes and extrudes Cu(I) independently.
