EMD-22939
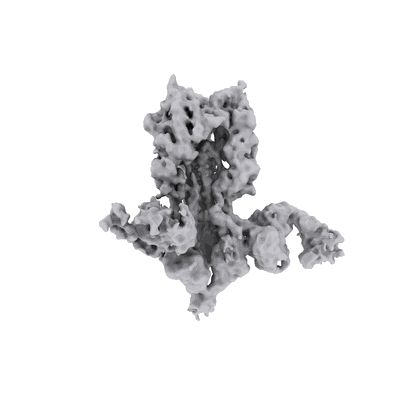
H5 hemagglutinin ectodomain bound to 2 polyclonal Fab fragments elicited by the qsMosaic-I53_dn5 immunogen
EMD-22939
Single-particle4.1 Å
 Deposition: 04/11/2020
Deposition: 04/11/2020Map released: 07/04/2021
Last modified: 05/05/2021
Sample Organism:
unidentified influenza virus,
Macaca mulatta
Sample: H5 hemagglutinin ectodomain bound to 2 polyclonal Fab fragments elicited by the qsMosaic-I53_dn5 immunogen
Deposition Authors: Park YJ, Veesler D
Sample: H5 hemagglutinin ectodomain bound to 2 polyclonal Fab fragments elicited by the qsMosaic-I53_dn5 immunogen
Deposition Authors: Park YJ, Veesler D
Quadrivalent influenza nanoparticle vaccines induce broad protection.
Boyoglu-Barnum S,
Ellis D,
Gillespie RA,
Hutchinson GB  ,
Park YJ
,
Park YJ  ,
Moin SM,
Acton OJ
,
Moin SM,
Acton OJ  ,
Ravichandran R,
Murphy M
,
Ravichandran R,
Murphy M  ,
Pettie D
,
Pettie D  ,
Matheson N,
Carter L,
Creanga A,
Watson MJ,
Kephart S
,
Matheson N,
Carter L,
Creanga A,
Watson MJ,
Kephart S  ,
Ataca S,
Vaile JR,
Ueda G
,
Ataca S,
Vaile JR,
Ueda G  ,
Crank MC,
Stewart L
,
Crank MC,
Stewart L  ,
Lee KK,
Guttman M,
Baker D
,
Lee KK,
Guttman M,
Baker D  ,
Mascola JR,
Veesler D
,
Mascola JR,
Veesler D  ,
Graham BS
,
Graham BS  ,
King NP
,
King NP  ,
Kanekiyo M
,
Kanekiyo M 
(2021) Nature , 592 , 623 - 628
 ,
Park YJ
,
Park YJ  ,
Moin SM,
Acton OJ
,
Moin SM,
Acton OJ  ,
Ravichandran R,
Murphy M
,
Ravichandran R,
Murphy M  ,
Pettie D
,
Pettie D  ,
Matheson N,
Carter L,
Creanga A,
Watson MJ,
Kephart S
,
Matheson N,
Carter L,
Creanga A,
Watson MJ,
Kephart S  ,
Ataca S,
Vaile JR,
Ueda G
,
Ataca S,
Vaile JR,
Ueda G  ,
Crank MC,
Stewart L
,
Crank MC,
Stewart L  ,
Lee KK,
Guttman M,
Baker D
,
Lee KK,
Guttman M,
Baker D  ,
Mascola JR,
Veesler D
,
Mascola JR,
Veesler D  ,
Graham BS
,
Graham BS  ,
King NP
,
King NP  ,
Kanekiyo M
,
Kanekiyo M 
(2021) Nature , 592 , 623 - 628
Abstract:
Influenza vaccines that confer broad and durable protection against diverse viral strains would have a major effect on global health, as they would lessen the need for annual vaccine reformulation and immunization1. Here we show that computationally designed, two-component nanoparticle immunogens2 induce potently neutralizing and broadly protective antibody responses against a wide variety of influenza viruses. The nanoparticle immunogens contain 20 haemagglutinin glycoprotein trimers in an ordered array, and their assembly in vitro enables the precisely controlled co-display of multiple distinct haemagglutinin proteins in defined ratios. Nanoparticle immunogens that co-display the four haemagglutinins of licensed quadrivalent influenza vaccines elicited antibody responses in several animal models against vaccine-matched strains that were equivalent to or better than commercial quadrivalent influenza vaccines, and simultaneously induced broadly protective antibody responses to heterologous viruses by targeting the subdominant yet conserved haemagglutinin stem. The combination of potent receptor-blocking and cross-reactive stem-directed antibodies induced by the nanoparticle immunogens makes them attractive candidates for a supraseasonal influenza vaccine candidate with the potential to replace conventional seasonal vaccines3.
Influenza vaccines that confer broad and durable protection against diverse viral strains would have a major effect on global health, as they would lessen the need for annual vaccine reformulation and immunization1. Here we show that computationally designed, two-component nanoparticle immunogens2 induce potently neutralizing and broadly protective antibody responses against a wide variety of influenza viruses. The nanoparticle immunogens contain 20 haemagglutinin glycoprotein trimers in an ordered array, and their assembly in vitro enables the precisely controlled co-display of multiple distinct haemagglutinin proteins in defined ratios. Nanoparticle immunogens that co-display the four haemagglutinins of licensed quadrivalent influenza vaccines elicited antibody responses in several animal models against vaccine-matched strains that were equivalent to or better than commercial quadrivalent influenza vaccines, and simultaneously induced broadly protective antibody responses to heterologous viruses by targeting the subdominant yet conserved haemagglutinin stem. The combination of potent receptor-blocking and cross-reactive stem-directed antibodies induced by the nanoparticle immunogens makes them attractive candidates for a supraseasonal influenza vaccine candidate with the potential to replace conventional seasonal vaccines3.
