EMD-2500
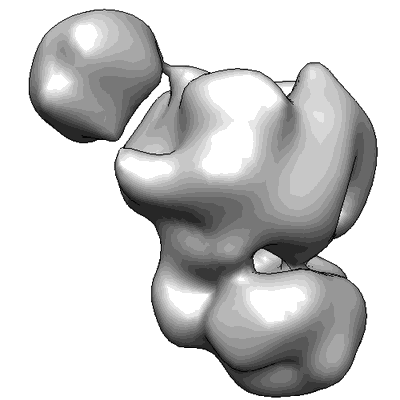
Substrate Recruitment Pathways in the Yeast Exosome by Electron Microscopy
EMD-2500
Single-particle25.0 Å
 Deposition: 23/10/2013
Deposition: 23/10/2013Map released: 25/12/2013
Last modified: 12/10/2016
Sample Organism:
Saccharomyces cerevisiae,
synthetic construct,
Escherichia coli
Sample: Rrp44-Exosome with Streptavidin labeled RNA50
Deposition Authors: Liu J-J, Bratkowski MA, Liu XQ, Niu C-Y, Ke AL, Wang H-W
Sample: Rrp44-Exosome with Streptavidin labeled RNA50
Deposition Authors: Liu J-J, Bratkowski MA, Liu XQ, Niu C-Y, Ke AL, Wang H-W
Visualization of distinct substrate-recruitment pathways in the yeast exosome by EM.
Abstract:
The eukaryotic exosome is a multisubunit complex typically composed of a catalytically inactive core and the Rrp44 protein, which contains 3'-to-5' exo- and endo-RNase activities. RNA substrates have been shown to be recruited through the core to reach Rrp44's exo-RNase (EXO) site. Using single-particle EM and biochemical analysis, we provide visual evidence that two distinct substrate-recruitment pathways exist. In the through-core route, channeling of the single-stranded substrates from the core to Rrp44 induces a characteristic conformational change in Rrp44. In the alternative direct-access route, this conformational change does not take place, and the RNA substrate is visualized to avoid the core and enter Rrp44's EXO site directly. Our results provide mechanistic explanations for several RNA processing scenarios by the eukaryotic exosome and indicate substrate-specific modes of degradation by this complex.
The eukaryotic exosome is a multisubunit complex typically composed of a catalytically inactive core and the Rrp44 protein, which contains 3'-to-5' exo- and endo-RNase activities. RNA substrates have been shown to be recruited through the core to reach Rrp44's exo-RNase (EXO) site. Using single-particle EM and biochemical analysis, we provide visual evidence that two distinct substrate-recruitment pathways exist. In the through-core route, channeling of the single-stranded substrates from the core to Rrp44 induces a characteristic conformational change in Rrp44. In the alternative direct-access route, this conformational change does not take place, and the RNA substrate is visualized to avoid the core and enter Rrp44's EXO site directly. Our results provide mechanistic explanations for several RNA processing scenarios by the eukaryotic exosome and indicate substrate-specific modes of degradation by this complex.
