EMD-25381
Composite map of ciliary C1 central pair apparatus isolated from Chlamydomonas reinhardtii
EMD-25381
Single-particle3.8 Å
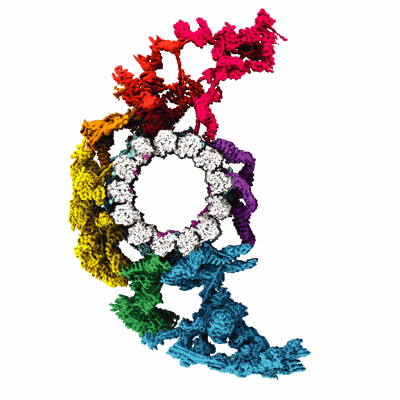 Deposition: 05/11/2021
Deposition: 05/11/2021Map released: 13/04/2022
Last modified: 05/06/2024
Sample Organism:
Chlamydomonas reinhardtii
Sample: C1 central pair apparatus complex
Fitted models: 7sqc
Deposition Authors: Gui M ,
Wang X,
Dutcher SK
,
Wang X,
Dutcher SK  ,
Brown A
,
Brown A  ,
Zhang R
,
Zhang R 
Sample: C1 central pair apparatus complex
Fitted models: 7sqc
Deposition Authors: Gui M
 ,
Wang X,
Dutcher SK
,
Wang X,
Dutcher SK  ,
Brown A
,
Brown A  ,
Zhang R
,
Zhang R 
Ciliary central apparatus structure reveals mechanisms of microtubule patterning.
Abstract:
A pair of extensively modified microtubules form the central apparatus (CA) of the axoneme of most motile cilia, where they regulate ciliary motility. The external surfaces of both CA microtubules are patterned asymmetrically with large protein complexes that repeat every 16 or 32 nm. The composition of these projections and the mechanisms that establish asymmetry and longitudinal periodicity are unknown. Here, by determining cryo-EM structures of the CA microtubules, we identify 48 different CA-associated proteins, which in turn reveal mechanisms for asymmetric and periodic protein binding to microtubules. We identify arc-MIPs, a novel class of microtubule inner protein, that bind laterally across protofilaments and remodel tubulin structure and lattice contacts. The binding mechanisms utilized by CA proteins may be generalizable to other microtubule-associated proteins. These structures establish a foundation to elucidate the contributions of individual CA proteins to ciliary motility and ciliopathies.
A pair of extensively modified microtubules form the central apparatus (CA) of the axoneme of most motile cilia, where they regulate ciliary motility. The external surfaces of both CA microtubules are patterned asymmetrically with large protein complexes that repeat every 16 or 32 nm. The composition of these projections and the mechanisms that establish asymmetry and longitudinal periodicity are unknown. Here, by determining cryo-EM structures of the CA microtubules, we identify 48 different CA-associated proteins, which in turn reveal mechanisms for asymmetric and periodic protein binding to microtubules. We identify arc-MIPs, a novel class of microtubule inner protein, that bind laterally across protofilaments and remodel tubulin structure and lattice contacts. The binding mechanisms utilized by CA proteins may be generalizable to other microtubule-associated proteins. These structures establish a foundation to elucidate the contributions of individual CA proteins to ciliary motility and ciliopathies.
