EMD-25738
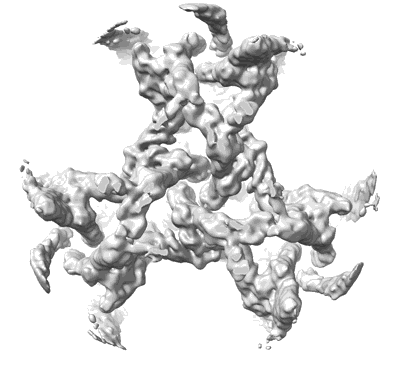
Subtomogram average of Munc13-1 C1-C2B-MUN-C2C trimer within 2D crystal between lipid bilayers.
EMD-25738
Subtomogram averaging10.0 Å
 Deposition: 15/12/2021
Deposition: 15/12/2021Map released: 09/02/2022
Last modified: 28/02/2024
Sample Organism:
Rattus norvegicus,
Mus musculus
Sample: 2D crystal of Munc13-1 C1-C2B-MUN-C2C domains between two lipid bilayers.
Fitted models: 7t7r (Avg. Q-score: 0.122)
Deposition Authors: Grushin K, Sindelar CV
Sample: 2D crystal of Munc13-1 C1-C2B-MUN-C2C domains between two lipid bilayers.
Fitted models: 7t7r (Avg. Q-score: 0.122)
Deposition Authors: Grushin K, Sindelar CV

Munc13 structural transitions and oligomers that may choreograph successive stages in vesicle priming for neurotransmitter release.
Abstract:
How can exactly six SNARE complexes be assembled under each synaptic vesicle? Here we report cryo-EM crystal structures of the core domain of Munc13, the key chaperone that initiates SNAREpin assembly. The functional core of Munc13, consisting of C1-C2B-MUN-C2C (Munc13C) spontaneously crystallizes between phosphatidylserine-rich bilayers in two distinct conformations, each in a radically different oligomeric state. In the open conformation (state 1), Munc13C forms upright trimers that link the two bilayers, separating them by ∼21 nm. In the closed conformation, six copies of Munc13C interact to form a lateral hexamer elevated ∼14 nm above the bilayer. Open and closed conformations differ only by a rigid body rotation around a flexible hinge, which when performed cooperatively assembles Munc13 into a lateral hexamer (state 2) in which the key SNARE assembly-activating site of Munc13 is autoinhibited by its neighbor. We propose that each Munc13 in the lateral hexamer ultimately assembles a single SNAREpin, explaining how only and exactly six SNARE complexes are templated. We suggest that state 1 and state 2 may represent two successive states in the synaptic vesicle supply chain leading to "primed" ready-release vesicles in which SNAREpins are clamped and ready to release (state 3).
How can exactly six SNARE complexes be assembled under each synaptic vesicle? Here we report cryo-EM crystal structures of the core domain of Munc13, the key chaperone that initiates SNAREpin assembly. The functional core of Munc13, consisting of C1-C2B-MUN-C2C (Munc13C) spontaneously crystallizes between phosphatidylserine-rich bilayers in two distinct conformations, each in a radically different oligomeric state. In the open conformation (state 1), Munc13C forms upright trimers that link the two bilayers, separating them by ∼21 nm. In the closed conformation, six copies of Munc13C interact to form a lateral hexamer elevated ∼14 nm above the bilayer. Open and closed conformations differ only by a rigid body rotation around a flexible hinge, which when performed cooperatively assembles Munc13 into a lateral hexamer (state 2) in which the key SNARE assembly-activating site of Munc13 is autoinhibited by its neighbor. We propose that each Munc13 in the lateral hexamer ultimately assembles a single SNAREpin, explaining how only and exactly six SNARE complexes are templated. We suggest that state 1 and state 2 may represent two successive states in the synaptic vesicle supply chain leading to "primed" ready-release vesicles in which SNAREpins are clamped and ready to release (state 3).
