EMD-26617
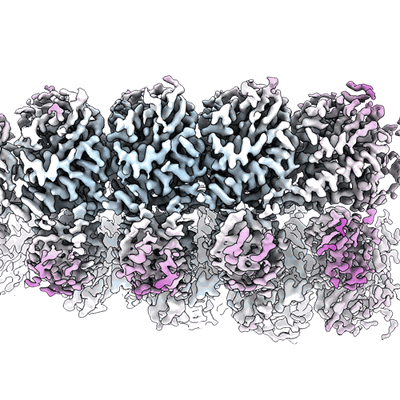
SfSTING with c-di-GMP double fiber
EMD-26617
Single-particle3.3 Å
 Deposition: 09/04/2022
Deposition: 09/04/2022Map released: 27/07/2022
Last modified: 14/02/2024
Sample Organism:
Sphingobacterium faecium
Sample: SfSTING with c-di-GMP double fiber
Fitted models: 7un9 (Avg. Q-score: 0.515)
Deposition Authors: Morehouse BR ,
Yip MCJ
,
Yip MCJ  ,
Keszei AFA
,
Keszei AFA  ,
McNamara-Bordewick NK
,
McNamara-Bordewick NK  ,
Shao S
,
Shao S  ,
Kranzusch PJ
,
Kranzusch PJ 
Sample: SfSTING with c-di-GMP double fiber
Fitted models: 7un9 (Avg. Q-score: 0.515)
Deposition Authors: Morehouse BR
 ,
Yip MCJ
,
Yip MCJ  ,
Keszei AFA
,
Keszei AFA  ,
McNamara-Bordewick NK
,
McNamara-Bordewick NK  ,
Shao S
,
Shao S  ,
Kranzusch PJ
,
Kranzusch PJ 
Cryo-EM structure of an active bacterial TIR-STING filament complex.
Morehouse BR  ,
Yip MCJ
,
Yip MCJ  ,
Keszei AFA
,
Keszei AFA  ,
McNamara-Bordewick NK
,
McNamara-Bordewick NK  ,
Shao S
,
Shao S  ,
Kranzusch PJ
,
Kranzusch PJ 
(2022) Nature , 608 , 803 - 807
 ,
Yip MCJ
,
Yip MCJ  ,
Keszei AFA
,
Keszei AFA  ,
McNamara-Bordewick NK
,
McNamara-Bordewick NK  ,
Shao S
,
Shao S  ,
Kranzusch PJ
,
Kranzusch PJ 
(2022) Nature , 608 , 803 - 807
Abstract:
Stimulator of interferon genes (STING) is an antiviral signalling protein that is broadly conserved in both innate immunity in animals and phage defence in prokaryotes1-4. Activation of STING requires its assembly into an oligomeric filament structure through binding of a cyclic dinucleotide4-13, but the molecular basis of STING filament assembly and extension remains unknown. Here we use cryogenic electron microscopy to determine the structure of the active Toll/interleukin-1 receptor (TIR)-STING filament complex from a Sphingobacterium faecium cyclic-oligonucleotide-based antiphage signalling system (CBASS) defence operon. Bacterial TIR-STING filament formation is driven by STING interfaces that become exposed on high-affinity recognition of the cognate cyclic dinucleotide signal c-di-GMP. Repeating dimeric STING units stack laterally head-to-head through surface interfaces, which are also essential for human STING tetramer formation and downstream immune signalling in mammals5. The active bacterial TIR-STING structure reveals further cross-filament contacts that brace the assembly and coordinate packing of the associated TIR NADase effector domains at the base of the filament to drive NAD+ hydrolysis. STING interface and cross-filament contacts are essential for cell growth arrest in vivo and reveal a stepwise mechanism of activation whereby STING filament assembly is required for subsequent effector activation. Our results define the structural basis of STING filament formation in prokaryotic antiviral signalling.
Stimulator of interferon genes (STING) is an antiviral signalling protein that is broadly conserved in both innate immunity in animals and phage defence in prokaryotes1-4. Activation of STING requires its assembly into an oligomeric filament structure through binding of a cyclic dinucleotide4-13, but the molecular basis of STING filament assembly and extension remains unknown. Here we use cryogenic electron microscopy to determine the structure of the active Toll/interleukin-1 receptor (TIR)-STING filament complex from a Sphingobacterium faecium cyclic-oligonucleotide-based antiphage signalling system (CBASS) defence operon. Bacterial TIR-STING filament formation is driven by STING interfaces that become exposed on high-affinity recognition of the cognate cyclic dinucleotide signal c-di-GMP. Repeating dimeric STING units stack laterally head-to-head through surface interfaces, which are also essential for human STING tetramer formation and downstream immune signalling in mammals5. The active bacterial TIR-STING structure reveals further cross-filament contacts that brace the assembly and coordinate packing of the associated TIR NADase effector domains at the base of the filament to drive NAD+ hydrolysis. STING interface and cross-filament contacts are essential for cell growth arrest in vivo and reveal a stepwise mechanism of activation whereby STING filament assembly is required for subsequent effector activation. Our results define the structural basis of STING filament formation in prokaryotic antiviral signalling.
