EMD-27203
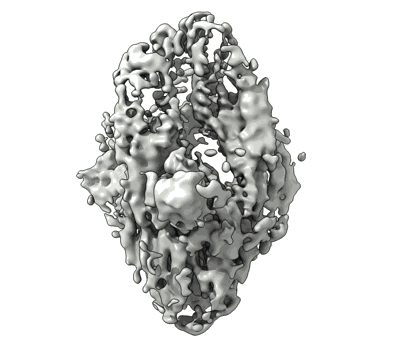
IMM20190 Fab complex with SARS-CoV-2 Spike Trimer
EMD-27203
Single-particle6.87 Å
 Deposition: 03/06/2022
Deposition: 03/06/2022Map released: 13/07/2022
Last modified: 17/01/2024
Sample Organism:
Severe acute respiratory syndrome coronavirus 2
Sample: IMM20253 Fab complex with REF Spike Trimer
Deposition Authors: Nikitin PA ,
DiMuzio JD,
Dowling JP
,
DiMuzio JD,
Dowling JP  ,
Patel NB
,
Patel NB  ,
Bingaman-Steele JL
,
Bingaman-Steele JL  ,
Heimbach BC
,
Heimbach BC  ,
Henriquez N
,
Henriquez N  ,
Nicolescu C
,
Nicolescu C  ,
Polley A
,
Polley A  ,
Sikorski EL,
Howanski RJ,
Nath M
,
Sikorski EL,
Howanski RJ,
Nath M  ,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF
,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF  ,
Smith T,
Storm N
,
Smith T,
Storm N  ,
McKay LGA
,
McKay LGA  ,
Johnson RI
,
Johnson RI  ,
Malsick LE
,
Malsick LE  ,
Honko AN
,
Honko AN  ,
Griffiths A
,
Griffiths A  ,
Diamond MS
,
Diamond MS  ,
Sarma P
,
Sarma P  ,
Geising DH
,
Geising DH  ,
Morin MJ,
Robinson MK
,
Morin MJ,
Robinson MK 
Sample: IMM20253 Fab complex with REF Spike Trimer
Deposition Authors: Nikitin PA
 ,
DiMuzio JD,
Dowling JP
,
DiMuzio JD,
Dowling JP  ,
Patel NB
,
Patel NB  ,
Bingaman-Steele JL
,
Bingaman-Steele JL  ,
Heimbach BC
,
Heimbach BC  ,
Henriquez N
,
Henriquez N  ,
Nicolescu C
,
Nicolescu C  ,
Polley A
,
Polley A  ,
Sikorski EL,
Howanski RJ,
Nath M
,
Sikorski EL,
Howanski RJ,
Nath M  ,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF
,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF  ,
Smith T,
Storm N
,
Smith T,
Storm N  ,
McKay LGA
,
McKay LGA  ,
Johnson RI
,
Johnson RI  ,
Malsick LE
,
Malsick LE  ,
Honko AN
,
Honko AN  ,
Griffiths A
,
Griffiths A  ,
Diamond MS
,
Diamond MS  ,
Sarma P
,
Sarma P  ,
Geising DH
,
Geising DH  ,
Morin MJ,
Robinson MK
,
Morin MJ,
Robinson MK 
IMM-BCP-01, a patient-derived anti-SARS-CoV-2 antibody cocktail, is active across variants of concern including Omicron BA.1 and BA.2.
Nikitin PA  ,
DiMuzio JM
,
DiMuzio JM  ,
Dowling JP
,
Dowling JP  ,
Patel NB
,
Patel NB  ,
Bingaman-Steele JL
,
Bingaman-Steele JL  ,
Heimbach BC
,
Heimbach BC  ,
Henriquez N
,
Henriquez N  ,
Nicolescu C
,
Nicolescu C  ,
Polley A
,
Polley A  ,
Sikorski EL,
Howanski RJ,
Nath M
,
Sikorski EL,
Howanski RJ,
Nath M  ,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF
,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF  ,
Smith T,
Storm N
,
Smith T,
Storm N  ,
McKay LGA
,
McKay LGA  ,
Johnson RI
,
Johnson RI  ,
Malsick LE
,
Malsick LE  ,
Honko AN
,
Honko AN  ,
Griffiths A
,
Griffiths A  ,
Diamond MS
,
Diamond MS  ,
Sarma P
,
Sarma P  ,
Geising DH
,
Geising DH  ,
Morin MJ,
Robinson MK
,
Morin MJ,
Robinson MK 
(2022) Sci Immunol , 7 , eabl9943 - eabl9943
 ,
DiMuzio JM
,
DiMuzio JM  ,
Dowling JP
,
Dowling JP  ,
Patel NB
,
Patel NB  ,
Bingaman-Steele JL
,
Bingaman-Steele JL  ,
Heimbach BC
,
Heimbach BC  ,
Henriquez N
,
Henriquez N  ,
Nicolescu C
,
Nicolescu C  ,
Polley A
,
Polley A  ,
Sikorski EL,
Howanski RJ,
Nath M
,
Sikorski EL,
Howanski RJ,
Nath M  ,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF
,
Shukla H,
Scheaffer SM,
Finn JP,
Liang LF  ,
Smith T,
Storm N
,
Smith T,
Storm N  ,
McKay LGA
,
McKay LGA  ,
Johnson RI
,
Johnson RI  ,
Malsick LE
,
Malsick LE  ,
Honko AN
,
Honko AN  ,
Griffiths A
,
Griffiths A  ,
Diamond MS
,
Diamond MS  ,
Sarma P
,
Sarma P  ,
Geising DH
,
Geising DH  ,
Morin MJ,
Robinson MK
,
Morin MJ,
Robinson MK 
(2022) Sci Immunol , 7 , eabl9943 - eabl9943
Abstract:
Monoclonal antibodies are an efficacious therapy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, rapid viral mutagenesis led to escape from most of these therapies, outlining the need for an antibody cocktail with a broad neutralizing potency. Using an unbiased interrogation of the memory B cell repertoire of patients with convalescent COVID-19, we identified human antibodies with broad antiviral activity in vitro and efficacy in vivo against all tested SARS-CoV-2 variants of concern, including Delta and Omicron BA.1 and BA.2. Here, we describe an antibody cocktail, IMM-BCP-01, that consists of three patient-derived broadly neutralizing antibodies directed at nonoverlapping surfaces on the SARS-CoV-2 Spike protein. Two antibodies, IMM20184 and IMM20190, directly blocked Spike binding to the ACE2 receptor. Binding of the third antibody, IMM20253, to its cryptic epitope on the outer surface of RBD altered the conformation of the Spike Trimer, promoting the release of Spike monomers. These antibodies decreased Omicron SARS-CoV-2 infection in the lungs of Syrian golden hamsters in vivo and potently induced antiviral effector response in vitro, including phagocytosis, ADCC, and complement pathway activation. Our preclinical data demonstrated that the three-antibody cocktail IMM-BCP-01 could be a promising means for preventing or treating infection of SARS-CoV-2 variants of concern, including Omicron BA.1 and BA.2, in susceptible individuals.
Monoclonal antibodies are an efficacious therapy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, rapid viral mutagenesis led to escape from most of these therapies, outlining the need for an antibody cocktail with a broad neutralizing potency. Using an unbiased interrogation of the memory B cell repertoire of patients with convalescent COVID-19, we identified human antibodies with broad antiviral activity in vitro and efficacy in vivo against all tested SARS-CoV-2 variants of concern, including Delta and Omicron BA.1 and BA.2. Here, we describe an antibody cocktail, IMM-BCP-01, that consists of three patient-derived broadly neutralizing antibodies directed at nonoverlapping surfaces on the SARS-CoV-2 Spike protein. Two antibodies, IMM20184 and IMM20190, directly blocked Spike binding to the ACE2 receptor. Binding of the third antibody, IMM20253, to its cryptic epitope on the outer surface of RBD altered the conformation of the Spike Trimer, promoting the release of Spike monomers. These antibodies decreased Omicron SARS-CoV-2 infection in the lungs of Syrian golden hamsters in vivo and potently induced antiviral effector response in vitro, including phagocytosis, ADCC, and complement pathway activation. Our preclinical data demonstrated that the three-antibody cocktail IMM-BCP-01 could be a promising means for preventing or treating infection of SARS-CoV-2 variants of concern, including Omicron BA.1 and BA.2, in susceptible individuals.
