EMD-27451
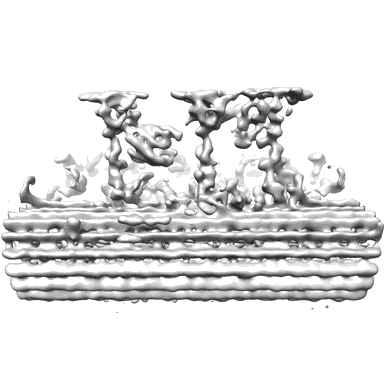
96-nm repeating structure of microtubule doublet 6 from mouse sperm
EMD-27451
Subtomogram averaging35.0 Å
 Deposition: 30/06/2022
Deposition: 30/06/2022Map released: 18/01/2023
Last modified: 29/03/2023
Sample Organism:
Mus musculus
Sample: Microtubule doublet from mouse sperm
Deposition Authors: Chen Z ,
Greenan AG,
Shiozak M,
Liu Y
,
Greenan AG,
Shiozak M,
Liu Y  ,
Skinner W,
Zhao X,
Zhao S,
Yan R,
Guo C,
Yu Z
,
Skinner W,
Zhao X,
Zhao S,
Yan R,
Guo C,
Yu Z  ,
Lishko PV
,
Lishko PV  ,
Agard DA
,
Agard DA  ,
Vale RD
,
Vale RD 
Sample: Microtubule doublet from mouse sperm
Deposition Authors: Chen Z
 ,
Greenan AG,
Shiozak M,
Liu Y
,
Greenan AG,
Shiozak M,
Liu Y  ,
Skinner W,
Zhao X,
Zhao S,
Yan R,
Guo C,
Yu Z
,
Skinner W,
Zhao X,
Zhao S,
Yan R,
Guo C,
Yu Z  ,
Lishko PV
,
Lishko PV  ,
Agard DA
,
Agard DA  ,
Vale RD
,
Vale RD 
In situ cryo-electron tomography reveals the asymmetric architecture of mammalian sperm axonemes.
Chen Z  ,
Greenan GA,
Shiozaki M,
Liu Y
,
Greenan GA,
Shiozaki M,
Liu Y  ,
Skinner WM
,
Skinner WM  ,
Zhao X,
Zhao S,
Yan R,
Yu Z
,
Zhao X,
Zhao S,
Yan R,
Yu Z  ,
Lishko PV
,
Lishko PV  ,
Agard DA
,
Agard DA  ,
Vale RD
,
Vale RD 
(2023) Nat Struct Mol Biol , 30 , 360 - 369
 ,
Greenan GA,
Shiozaki M,
Liu Y
,
Greenan GA,
Shiozaki M,
Liu Y  ,
Skinner WM
,
Skinner WM  ,
Zhao X,
Zhao S,
Yan R,
Yu Z
,
Zhao X,
Zhao S,
Yan R,
Yu Z  ,
Lishko PV
,
Lishko PV  ,
Agard DA
,
Agard DA  ,
Vale RD
,
Vale RD 
(2023) Nat Struct Mol Biol , 30 , 360 - 369
Abstract:
The flagella of mammalian sperm display non-planar, asymmetric beating, in contrast to the planar, symmetric beating of flagella from sea urchin sperm and unicellular organisms. The molecular basis of this difference is unclear. Here, we perform in situ cryo-electron tomography of mouse and human sperm, providing the highest-resolution structural information to date. Our subtomogram averages reveal mammalian sperm-specific protein complexes within the microtubules, the radial spokes and nexin-dynein regulatory complexes. The locations and structures of these complexes suggest potential roles in enhancing the mechanical strength of mammalian sperm axonemes and regulating dynein-based axonemal bending. Intriguingly, we find that each of the nine outer microtubule doublets is decorated with a distinct combination of sperm-specific complexes. We propose that this asymmetric distribution of proteins differentially regulates the sliding of each microtubule doublet and may underlie the asymmetric beating of mammalian sperm.
The flagella of mammalian sperm display non-planar, asymmetric beating, in contrast to the planar, symmetric beating of flagella from sea urchin sperm and unicellular organisms. The molecular basis of this difference is unclear. Here, we perform in situ cryo-electron tomography of mouse and human sperm, providing the highest-resolution structural information to date. Our subtomogram averages reveal mammalian sperm-specific protein complexes within the microtubules, the radial spokes and nexin-dynein regulatory complexes. The locations and structures of these complexes suggest potential roles in enhancing the mechanical strength of mammalian sperm axonemes and regulating dynein-based axonemal bending. Intriguingly, we find that each of the nine outer microtubule doublets is decorated with a distinct combination of sperm-specific complexes. We propose that this asymmetric distribution of proteins differentially regulates the sliding of each microtubule doublet and may underlie the asymmetric beating of mammalian sperm.
