EMD-28233
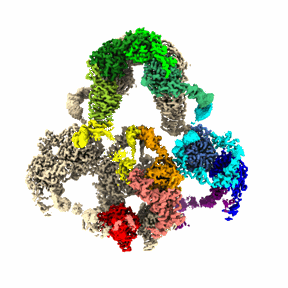
Cryo-EM structure of LRP2 at pH 7.5
EMD-28233
Single-particle2.83 Å
 Deposition: 26/09/2022
Deposition: 26/09/2022Map released: 08/02/2023
Last modified: 13/11/2024
Sample Organism:
Mus musculus
Sample: LRP2 at neutral pH
Fitted models: 8em4 (Avg. Q-score: 0.36)
Deposition Authors: Beenken A ,
Cerutti G
,
Cerutti G  ,
Brasch J,
Fitzpatrick AW
,
Brasch J,
Fitzpatrick AW  ,
Barasch J
,
Barasch J  ,
Shapiro L
,
Shapiro L 
Sample: LRP2 at neutral pH
Fitted models: 8em4 (Avg. Q-score: 0.36)
Deposition Authors: Beenken A
 ,
Cerutti G
,
Cerutti G  ,
Brasch J,
Fitzpatrick AW
,
Brasch J,
Fitzpatrick AW  ,
Barasch J
,
Barasch J  ,
Shapiro L
,
Shapiro L 
Structures of LRP2 reveal a molecular machine for endocytosis.
Beenken A  ,
Cerutti G
,
Cerutti G  ,
Brasch J,
Guo Y,
Sheng Z,
Erdjument-Bromage H
,
Brasch J,
Guo Y,
Sheng Z,
Erdjument-Bromage H  ,
Aziz Z,
Robbins-Juarez SY,
Chavez EY,
Ahlsen G,
Katsamba PS
,
Aziz Z,
Robbins-Juarez SY,
Chavez EY,
Ahlsen G,
Katsamba PS  ,
Neubert TA,
Fitzpatrick AWP,
Barasch J
,
Neubert TA,
Fitzpatrick AWP,
Barasch J  ,
Shapiro L
,
Shapiro L 
(2023) Cell , 186 , 821
 ,
Cerutti G
,
Cerutti G  ,
Brasch J,
Guo Y,
Sheng Z,
Erdjument-Bromage H
,
Brasch J,
Guo Y,
Sheng Z,
Erdjument-Bromage H  ,
Aziz Z,
Robbins-Juarez SY,
Chavez EY,
Ahlsen G,
Katsamba PS
,
Aziz Z,
Robbins-Juarez SY,
Chavez EY,
Ahlsen G,
Katsamba PS  ,
Neubert TA,
Fitzpatrick AWP,
Barasch J
,
Neubert TA,
Fitzpatrick AWP,
Barasch J  ,
Shapiro L
,
Shapiro L 
(2023) Cell , 186 , 821
Abstract:
The low-density lipoprotein (LDL) receptor-related protein 2 (LRP2 or megalin) is representative of the phylogenetically conserved subfamily of giant LDL receptor-related proteins, which function in endocytosis and are implicated in diseases of the kidney and brain. Here, we report high-resolution cryoelectron microscopy structures of LRP2 isolated from mouse kidney, at extracellular and endosomal pH. The structures reveal LRP2 to be a molecular machine that adopts a conformation for ligand binding at the cell surface and for ligand shedding in the endosome. LRP2 forms a homodimer, the conformational transformation of which is governed by pH-sensitive sites at both homodimer and intra-protomer interfaces. A subset of LRP2 deleterious missense variants in humans appears to impair homodimer assembly. These observations lay the foundation for further understanding the function and mechanism of LDL receptors and implicate homodimerization as a conserved feature of the LRP receptor subfamily.
The low-density lipoprotein (LDL) receptor-related protein 2 (LRP2 or megalin) is representative of the phylogenetically conserved subfamily of giant LDL receptor-related proteins, which function in endocytosis and are implicated in diseases of the kidney and brain. Here, we report high-resolution cryoelectron microscopy structures of LRP2 isolated from mouse kidney, at extracellular and endosomal pH. The structures reveal LRP2 to be a molecular machine that adopts a conformation for ligand binding at the cell surface and for ligand shedding in the endosome. LRP2 forms a homodimer, the conformational transformation of which is governed by pH-sensitive sites at both homodimer and intra-protomer interfaces. A subset of LRP2 deleterious missense variants in humans appears to impair homodimer assembly. These observations lay the foundation for further understanding the function and mechanism of LDL receptors and implicate homodimerization as a conserved feature of the LRP receptor subfamily.
