EMD-28571
CryoEM structure of PN45545 TCR-CD3 in complex with HLA-A2 MAGEA4 (230-239)
EMD-28571
Single-particle2.65 Å
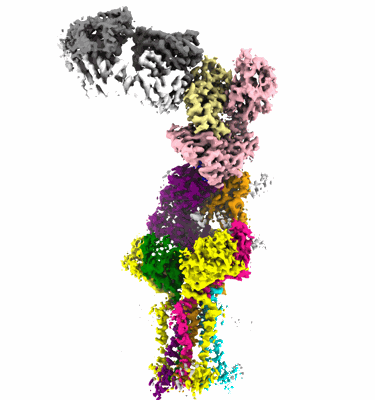 Deposition: 13/10/2022
Deposition: 13/10/2022Map released: 03/05/2023
Last modified: 20/11/2024
Sample Organism:
Homo sapiens
Sample: PN45545 TCR-CD3 complex bound to HLA-A2 MAGEA4 (230-239) with anti-Beta-2-microglobulin Fab
Fitted models: 8es8 (Avg. Q-score: 0.537)
Deposition Authors: Saotome K ,
Franklin MC
,
Franklin MC 
Sample: PN45545 TCR-CD3 complex bound to HLA-A2 MAGEA4 (230-239) with anti-Beta-2-microglobulin Fab
Fitted models: 8es8 (Avg. Q-score: 0.537)
Deposition Authors: Saotome K
 ,
Franklin MC
,
Franklin MC 
Structural analysis of cancer-relevant TCR-CD3 and peptide-MHC complexes by cryoEM.
Saotome K  ,
Dudgeon D
,
Dudgeon D  ,
Colotti K,
Moore MJ,
Jones J,
Zhou Y,
Rafique A
,
Colotti K,
Moore MJ,
Jones J,
Zhou Y,
Rafique A  ,
Yancopoulos GD,
Murphy AJ
,
Yancopoulos GD,
Murphy AJ  ,
Lin JC,
Olson WC,
Franklin MC
,
Lin JC,
Olson WC,
Franklin MC 
(2023) Nat Commun , 14 , 2401 - 2401
 ,
Dudgeon D
,
Dudgeon D  ,
Colotti K,
Moore MJ,
Jones J,
Zhou Y,
Rafique A
,
Colotti K,
Moore MJ,
Jones J,
Zhou Y,
Rafique A  ,
Yancopoulos GD,
Murphy AJ
,
Yancopoulos GD,
Murphy AJ  ,
Lin JC,
Olson WC,
Franklin MC
,
Lin JC,
Olson WC,
Franklin MC 
(2023) Nat Commun , 14 , 2401 - 2401
Abstract:
The recognition of antigenic peptide-MHC (pMHC) molecules by T-cell receptors (TCR) initiates the T-cell mediated immune response. Structural characterization is key for understanding the specificity of TCR-pMHC interactions and informing the development of therapeutics. Despite the rapid rise of single particle cryoelectron microscopy (cryoEM), x-ray crystallography has remained the preferred method for structure determination of TCR-pMHC complexes. Here, we report cryoEM structures of two distinct full-length α/β TCR-CD3 complexes bound to their pMHC ligand, the cancer-testis antigen HLA-A2/MAGEA4 (230-239). We also determined cryoEM structures of pMHCs containing MAGEA4 (230-239) peptide and the closely related MAGEA8 (232-241) peptide in the absence of TCR, which provided a structural explanation for the MAGEA4 preference displayed by the TCRs. These findings provide insights into the TCR recognition of a clinically relevant cancer antigen and demonstrate the utility of cryoEM for high-resolution structural analysis of TCR-pMHC interactions.
The recognition of antigenic peptide-MHC (pMHC) molecules by T-cell receptors (TCR) initiates the T-cell mediated immune response. Structural characterization is key for understanding the specificity of TCR-pMHC interactions and informing the development of therapeutics. Despite the rapid rise of single particle cryoelectron microscopy (cryoEM), x-ray crystallography has remained the preferred method for structure determination of TCR-pMHC complexes. Here, we report cryoEM structures of two distinct full-length α/β TCR-CD3 complexes bound to their pMHC ligand, the cancer-testis antigen HLA-A2/MAGEA4 (230-239). We also determined cryoEM structures of pMHCs containing MAGEA4 (230-239) peptide and the closely related MAGEA8 (232-241) peptide in the absence of TCR, which provided a structural explanation for the MAGEA4 preference displayed by the TCRs. These findings provide insights into the TCR recognition of a clinically relevant cancer antigen and demonstrate the utility of cryoEM for high-resolution structural analysis of TCR-pMHC interactions.
