EMD-30325
Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody
EMD-30325
Single-particle3.52 Å
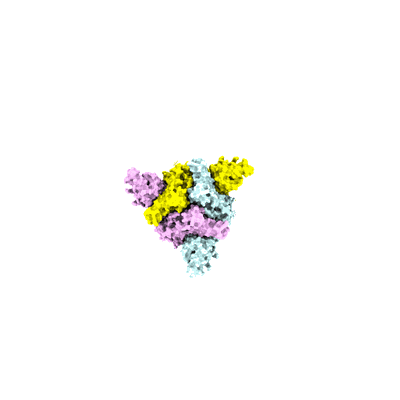 Deposition: 08/06/2020
Deposition: 08/06/2020Map released: 16/12/2020
Last modified: 16/10/2024
Sample Organism:
Severe acute respiratory syndrome coronavirus 2
Sample: SARS-CoV-2 spike glycoprotein trimer
Fitted models: 7cab (Avg. Q-score: 0.476)
Deposition Authors: Zhe L, Cao L
Sample: SARS-CoV-2 spike glycoprotein trimer
Fitted models: 7cab (Avg. Q-score: 0.476)
Deposition Authors: Zhe L, Cao L

Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody.
Lv Z  ,
Deng YQ
,
Deng YQ  ,
Ye Q
,
Ye Q  ,
Cao L
,
Cao L  ,
Sun CY
,
Sun CY  ,
Fan C
,
Fan C  ,
Huang W
,
Huang W  ,
Sun S
,
Sun S  ,
Sun Y
,
Sun Y  ,
Zhu L
,
Zhu L  ,
Chen Q
,
Chen Q  ,
Wang N
,
Wang N  ,
Nie J,
Cui Z
,
Nie J,
Cui Z  ,
Zhu D
,
Zhu D  ,
Shaw N
,
Shaw N  ,
Li XF
,
Li XF  ,
Li Q,
Xie L
,
Li Q,
Xie L  ,
Wang Y
,
Wang Y  ,
Rao Z
,
Rao Z  ,
Qin CF
,
Qin CF  ,
Wang X
,
Wang X 
(2020) Science , 369 , 1505 - 1509
 ,
Deng YQ
,
Deng YQ  ,
Ye Q
,
Ye Q  ,
Cao L
,
Cao L  ,
Sun CY
,
Sun CY  ,
Fan C
,
Fan C  ,
Huang W
,
Huang W  ,
Sun S
,
Sun S  ,
Sun Y
,
Sun Y  ,
Zhu L
,
Zhu L  ,
Chen Q
,
Chen Q  ,
Wang N
,
Wang N  ,
Nie J,
Cui Z
,
Nie J,
Cui Z  ,
Zhu D
,
Zhu D  ,
Shaw N
,
Shaw N  ,
Li XF
,
Li XF  ,
Li Q,
Xie L
,
Li Q,
Xie L  ,
Wang Y
,
Wang Y  ,
Rao Z
,
Rao Z  ,
Qin CF
,
Qin CF  ,
Wang X
,
Wang X 
(2020) Science , 369 , 1505 - 1509
Abstract:
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an unprecedented public health crisis. There are no approved vaccines or therapeutics for treating COVID-19. Here we report a humanized monoclonal antibody, H014, that efficiently neutralizes SARS-CoV-2 and SARS-CoV pseudoviruses as well as authentic SARS-CoV-2 at nanomolar concentrations by engaging the spike (S) receptor binding domain (RBD). H014 administration reduced SARS-CoV-2 titers in infected lungs and prevented pulmonary pathology in a human angiotensin-converting enzyme 2 mouse model. Cryo-electron microscopy characterization of the SARS-CoV-2 S trimer in complex with the H014 Fab fragment unveiled a previously uncharacterized conformational epitope, which was only accessible when the RBD was in an open conformation. Biochemical, cellular, virological, and structural studies demonstrated that H014 prevents attachment of SARS-CoV-2 to its host cell receptors. Epitope analysis of available neutralizing antibodies against SARS-CoV and SARS-CoV-2 uncovered broad cross-protective epitopes. Our results highlight a key role for antibody-based therapeutic interventions in the treatment of COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an unprecedented public health crisis. There are no approved vaccines or therapeutics for treating COVID-19. Here we report a humanized monoclonal antibody, H014, that efficiently neutralizes SARS-CoV-2 and SARS-CoV pseudoviruses as well as authentic SARS-CoV-2 at nanomolar concentrations by engaging the spike (S) receptor binding domain (RBD). H014 administration reduced SARS-CoV-2 titers in infected lungs and prevented pulmonary pathology in a human angiotensin-converting enzyme 2 mouse model. Cryo-electron microscopy characterization of the SARS-CoV-2 S trimer in complex with the H014 Fab fragment unveiled a previously uncharacterized conformational epitope, which was only accessible when the RBD was in an open conformation. Biochemical, cellular, virological, and structural studies demonstrated that H014 prevents attachment of SARS-CoV-2 to its host cell receptors. Epitope analysis of available neutralizing antibodies against SARS-CoV and SARS-CoV-2 uncovered broad cross-protective epitopes. Our results highlight a key role for antibody-based therapeutic interventions in the treatment of COVID-19.
