EMD-33062
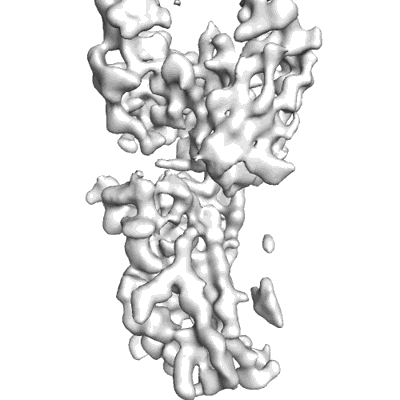
The SARS-CoV-2 receptor binding domain bound with the Fab fragment of a human neutralizing antibody Ab326
EMD-33062
Single-particle4.2 Å
 Deposition: 15/03/2022
Deposition: 15/03/2022Map released: 07/12/2022
Last modified: 16/10/2024
Sample Organism:
Homo sapiens,
Severe acute respiratory syndrome coronavirus 2
Sample: The SARS-CoV-2 spike protein bound with the Fab fragment of a human neutralizing antibody Ab326
Fitted models: 7x90 (Avg. Q-score: 0.392)
Deposition Authors: Kamada K ,
Shirouzu M
,
Shirouzu M
Sample: The SARS-CoV-2 spike protein bound with the Fab fragment of a human neutralizing antibody Ab326
Fitted models: 7x90 (Avg. Q-score: 0.392)
Deposition Authors: Kamada K
 ,
Shirouzu M
,
Shirouzu M
Potent SARS-CoV-2 neutralizing antibodies with therapeutic effects in two animal models.
Takeshita M  ,
Fukuyama H
,
Fukuyama H  ,
Kamada K
,
Kamada K  ,
Matsumoto T,
Makino-Okamura C,
Uchikubo-Kamo T,
Tomabechi Y
,
Matsumoto T,
Makino-Okamura C,
Uchikubo-Kamo T,
Tomabechi Y  ,
Hanada K,
Moriyama S,
Takahashi Y
,
Hanada K,
Moriyama S,
Takahashi Y  ,
Ishigaki H,
Nakayama M
,
Ishigaki H,
Nakayama M  ,
Nguyen CT,
Kitagawa Y,
Itoh Y,
Imai M,
Maemura T,
Furusawa Y,
Ueki H,
Iwatsuki-Horimoto K,
Ito M,
Yamayoshi S
,
Nguyen CT,
Kitagawa Y,
Itoh Y,
Imai M,
Maemura T,
Furusawa Y,
Ueki H,
Iwatsuki-Horimoto K,
Ito M,
Yamayoshi S  ,
Kawaoka Y,
Shirouzu M,
Ishii M
,
Kawaoka Y,
Shirouzu M,
Ishii M  ,
Saya H,
Kondo Y,
Kaneko Y,
Suzuki K,
Fukunaga K,
Takeuchi T
,
Saya H,
Kondo Y,
Kaneko Y,
Suzuki K,
Fukunaga K,
Takeuchi T
(2022) iScience , 25 , 105596 - 105596
 ,
Fukuyama H
,
Fukuyama H  ,
Kamada K
,
Kamada K  ,
Matsumoto T,
Makino-Okamura C,
Uchikubo-Kamo T,
Tomabechi Y
,
Matsumoto T,
Makino-Okamura C,
Uchikubo-Kamo T,
Tomabechi Y  ,
Hanada K,
Moriyama S,
Takahashi Y
,
Hanada K,
Moriyama S,
Takahashi Y  ,
Ishigaki H,
Nakayama M
,
Ishigaki H,
Nakayama M  ,
Nguyen CT,
Kitagawa Y,
Itoh Y,
Imai M,
Maemura T,
Furusawa Y,
Ueki H,
Iwatsuki-Horimoto K,
Ito M,
Yamayoshi S
,
Nguyen CT,
Kitagawa Y,
Itoh Y,
Imai M,
Maemura T,
Furusawa Y,
Ueki H,
Iwatsuki-Horimoto K,
Ito M,
Yamayoshi S  ,
Kawaoka Y,
Shirouzu M,
Ishii M
,
Kawaoka Y,
Shirouzu M,
Ishii M  ,
Saya H,
Kondo Y,
Kaneko Y,
Suzuki K,
Fukunaga K,
Takeuchi T
,
Saya H,
Kondo Y,
Kaneko Y,
Suzuki K,
Fukunaga K,
Takeuchi T
(2022) iScience , 25 , 105596 - 105596
Abstract:
The use of therapeutic neutralizing antibodies against SARS-CoV-2 infection has been highly effective. However, there remain few practical antibodies against viruses that are acquiring mutations. In this study, we created 494 monoclonal antibodies from patients with COVID-19-convalescent, and identified antibodies that exhibited the comparable neutralizing ability to clinically used antibodies in the neutralization assay using pseudovirus and authentic virus including variants of concerns. These antibodies have different profiles against various mutations, which were confirmed by cell-based assay and cryo-electron microscopy. To prevent antibody-dependent enhancement, N297A modification was introduced. Our antibodies showed a reduction of lung viral RNAs by therapeutic administration in a hamster model. In addition, an antibody cocktail consisting of three antibodies was also administered therapeutically to a macaque model, which resulted in reduced viral titers of swabs and lungs and reduced lung tissue damage scores. These results showed that our antibodies have sufficient antiviral activity as therapeutic candidates.
The use of therapeutic neutralizing antibodies against SARS-CoV-2 infection has been highly effective. However, there remain few practical antibodies against viruses that are acquiring mutations. In this study, we created 494 monoclonal antibodies from patients with COVID-19-convalescent, and identified antibodies that exhibited the comparable neutralizing ability to clinically used antibodies in the neutralization assay using pseudovirus and authentic virus including variants of concerns. These antibodies have different profiles against various mutations, which were confirmed by cell-based assay and cryo-electron microscopy. To prevent antibody-dependent enhancement, N297A modification was introduced. Our antibodies showed a reduction of lung viral RNAs by therapeutic administration in a hamster model. In addition, an antibody cocktail consisting of three antibodies was also administered therapeutically to a macaque model, which resulted in reduced viral titers of swabs and lungs and reduced lung tissue damage scores. These results showed that our antibodies have sufficient antiviral activity as therapeutic candidates.
