EMD-3310
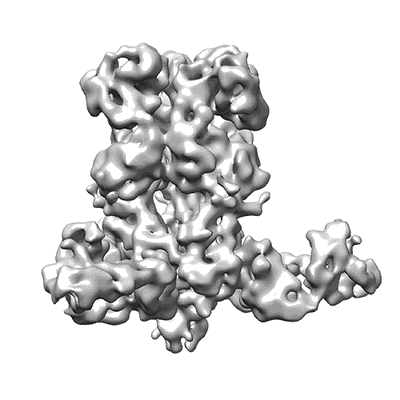
Cryo-EM structure of Ebola GP-Fab100-Fab114 ternary complex at pH 7.4
EMD-3310
Single-particle7.2 Å
 Deposition: 29/01/2016
Deposition: 29/01/2016Map released: 02/03/2016
Last modified: 13/04/2016
Sample Organism:
Homo sapiens,
Zaire ebolavirus
Sample: Ebola GP binds to Fab Fragments of mAb100 and mAb114
Deposition Authors: Misasi J ,
Gilman M
,
Gilman M  ,
Kanekiyo M
,
Kanekiyo M  ,
Gui M
,
Gui M  ,
Cagigi A
,
Cagigi A  ,
Mulangu S,
Corti D
,
Mulangu S,
Corti D  ,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U
,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U  ,
Graham B,
Xiang Y
,
Graham B,
Xiang Y  ,
Sullivan N,
McLellan J
,
Sullivan N,
McLellan J 
Sample: Ebola GP binds to Fab Fragments of mAb100 and mAb114
Deposition Authors: Misasi J
 ,
Gilman M
,
Gilman M  ,
Kanekiyo M
,
Kanekiyo M  ,
Gui M
,
Gui M  ,
Cagigi A
,
Cagigi A  ,
Mulangu S,
Corti D
,
Mulangu S,
Corti D  ,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U
,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U  ,
Graham B,
Xiang Y
,
Graham B,
Xiang Y  ,
Sullivan N,
McLellan J
,
Sullivan N,
McLellan J 
Structural and Molecular Basis for Ebola Virus Neutralization by Protective Human Antibodies
Misasi J  ,
Gilman M
,
Gilman M  ,
Kanekiyo M
,
Kanekiyo M  ,
Gui M
,
Gui M  ,
Cagigi A
,
Cagigi A  ,
Mulangu S,
Corti D
,
Mulangu S,
Corti D  ,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U
,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U  ,
Graham B,
Xiang Y
,
Graham B,
Xiang Y  ,
Sullivan N,
McLellan J
,
Sullivan N,
McLellan J 
(2016) Science , 351 , 1343 - 1346
 ,
Gilman M
,
Gilman M  ,
Kanekiyo M
,
Kanekiyo M  ,
Gui M
,
Gui M  ,
Cagigi A
,
Cagigi A  ,
Mulangu S,
Corti D
,
Mulangu S,
Corti D  ,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U
,
Ledgerwood J,
Lanzavecchia A,
Cunningham J,
Muyembe-Tamfun J,
Baxa U  ,
Graham B,
Xiang Y
,
Graham B,
Xiang Y  ,
Sullivan N,
McLellan J
,
Sullivan N,
McLellan J 
(2016) Science , 351 , 1343 - 1346
Abstract:
Ebola virus causes hemorrhagic fever with a high case fatality rate for which there is no approved therapy. Two human monoclonal antibodies, mAb100 and mAb114, in combination, protect nonhuman primates against all signs of Ebola virus disease, including viremia. Here, we demonstrate that mAb100 recognizes the base of the Ebola virus glycoprotein (GP) trimer, occludes access to the cathepsin-cleavage loop, and prevents the proteolytic cleavage of GP that is required for virus entry. We show that mAb114 interacts with the glycan cap and inner chalice of GP, remains associated after proteolytic removal of the glycan cap, and inhibits binding of cleaved GP to its receptor. These results define the basis of neutralization for two protective antibodies and may facilitate development of therapies and vaccines.
Ebola virus causes hemorrhagic fever with a high case fatality rate for which there is no approved therapy. Two human monoclonal antibodies, mAb100 and mAb114, in combination, protect nonhuman primates against all signs of Ebola virus disease, including viremia. Here, we demonstrate that mAb100 recognizes the base of the Ebola virus glycoprotein (GP) trimer, occludes access to the cathepsin-cleavage loop, and prevents the proteolytic cleavage of GP that is required for virus entry. We show that mAb114 interacts with the glycan cap and inner chalice of GP, remains associated after proteolytic removal of the glycan cap, and inhibits binding of cleaved GP to its receptor. These results define the basis of neutralization for two protective antibodies and may facilitate development of therapies and vaccines.
