EMD-33215
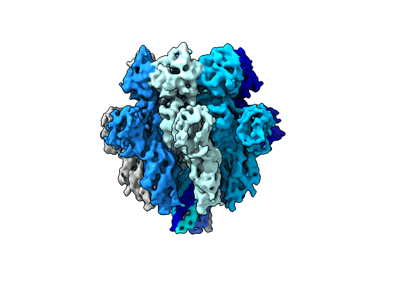
Cryo-EM reconstruction of complete transmembrane channel E289A mutant Vibrio cholerae Cytolysin
EMD-33215
Single-particle4.7 Å
 Deposition: 14/04/2022
Deposition: 14/04/2022Map released: 04/05/2022
Last modified: 09/10/2024
Sample Organism:
Vibrio cholerae
Sample: Cryo-EM reconstruction of complete transmembrane channel E289A mutant Vibrio cholerae Cytolysin
Fitted models: 7yl9
Deposition Authors: Mondal AK, Sengupta N, Singh M ,
Lata K,
Lahiri I
,
Lata K,
Lahiri I  ,
Dutta S
,
Dutta S  ,
Chattopadhyay K
,
Chattopadhyay K
Sample: Cryo-EM reconstruction of complete transmembrane channel E289A mutant Vibrio cholerae Cytolysin
Fitted models: 7yl9
Deposition Authors: Mondal AK, Sengupta N, Singh M
 ,
Lata K,
Lahiri I
,
Lata K,
Lahiri I  ,
Dutta S
,
Dutta S  ,
Chattopadhyay K
,
Chattopadhyay K
Glu289 residue in the pore-forming motif of Vibrio cholerae cytolysin is important for efficient beta-barrel pore formation.
Mondal AK,
Sengupta N,
Singh M  ,
Biswas R,
Lata K,
Lahiri I
,
Biswas R,
Lata K,
Lahiri I  ,
Dutta S
,
Dutta S  ,
Chattopadhyay K
,
Chattopadhyay K
(2022) J Biol Chem , 298 , 102441 - 102441
 ,
Biswas R,
Lata K,
Lahiri I
,
Biswas R,
Lata K,
Lahiri I  ,
Dutta S
,
Dutta S  ,
Chattopadhyay K
,
Chattopadhyay K
(2022) J Biol Chem , 298 , 102441 - 102441
Abstract:
Vibrio cholerae cytolysin (VCC) is a potent membrane-damaging β-barrel pore-forming toxin. Upon binding to the target membranes, VCC monomers first assemble into oligomeric prepore intermediates and subsequently transform into transmembrane β-barrel pores. VCC harbors a designated pore-forming motif, which, during oligomeric pore formation, inserts into the membrane and generates a transmembrane β-barrel scaffold. It remains an enigma how the molecular architecture of the pore-forming motif regulates the VCC pore-formation mechanism. Here, we show that a specific pore-forming motif residue, E289, plays crucial regulatory roles in the pore-formation mechanism of VCC. We find that the mutation of E289A drastically compromises pore-forming activity, without affecting the structural integrity and membrane-binding potential of the toxin monomers. Although our single-particle cryo-EM analysis reveals WT-like oligomeric β-barrel pore formation by E289A-VCC in the membrane, we demonstrate that the mutant shows severely delayed kinetics in terms of pore-forming ability that can be rescued with elevated temperature conditions. We find that the pore-formation efficacy of E289A-VCC appears to be more profoundly dependent on temperature than that of the WT toxin. Our results suggest that the E289A mutation traps membrane-bound toxin molecules in the prepore-like intermediate state that is hindered from converting into the functional β-barrel pores by a large energy barrier, thus highlighting the importance of this residue for the pore-formation mechanism of VCC.
Vibrio cholerae cytolysin (VCC) is a potent membrane-damaging β-barrel pore-forming toxin. Upon binding to the target membranes, VCC monomers first assemble into oligomeric prepore intermediates and subsequently transform into transmembrane β-barrel pores. VCC harbors a designated pore-forming motif, which, during oligomeric pore formation, inserts into the membrane and generates a transmembrane β-barrel scaffold. It remains an enigma how the molecular architecture of the pore-forming motif regulates the VCC pore-formation mechanism. Here, we show that a specific pore-forming motif residue, E289, plays crucial regulatory roles in the pore-formation mechanism of VCC. We find that the mutation of E289A drastically compromises pore-forming activity, without affecting the structural integrity and membrane-binding potential of the toxin monomers. Although our single-particle cryo-EM analysis reveals WT-like oligomeric β-barrel pore formation by E289A-VCC in the membrane, we demonstrate that the mutant shows severely delayed kinetics in terms of pore-forming ability that can be rescued with elevated temperature conditions. We find that the pore-formation efficacy of E289A-VCC appears to be more profoundly dependent on temperature than that of the WT toxin. Our results suggest that the E289A mutation traps membrane-bound toxin molecules in the prepore-like intermediate state that is hindered from converting into the functional β-barrel pores by a large energy barrier, thus highlighting the importance of this residue for the pore-formation mechanism of VCC.
