EMD-36078
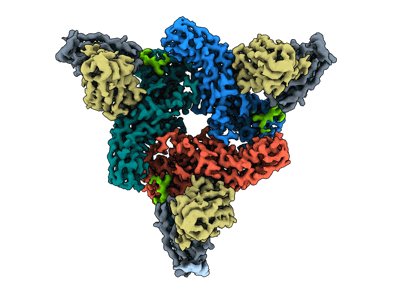
Structure of beta-arrestin2 in complex with M2Rpp
EMD-36078
Single-particle2.9 Å
 Deposition: 02/05/2023
Deposition: 02/05/2023Map released: 27/12/2023
Last modified: 13/11/2024
Sample Organism:
Mus musculus,
Bos taurus,
Homo sapiens
Sample: beta-arrestin2 in complex with M2Rpp
Fitted models: 8j8r (Avg. Q-score: 0.543)
Deposition Authors: Maharana J ,
Sano FK
,
Sano FK  ,
Shihoya W
,
Shihoya W  ,
Banerjee R
,
Banerjee R  ,
Nureki O
,
Nureki O  ,
Shukla AK
,
Shukla AK 
Sample: beta-arrestin2 in complex with M2Rpp
Fitted models: 8j8r (Avg. Q-score: 0.543)
Deposition Authors: Maharana J
 ,
Sano FK
,
Sano FK  ,
Shihoya W
,
Shihoya W  ,
Banerjee R
,
Banerjee R  ,
Nureki O
,
Nureki O  ,
Shukla AK
,
Shukla AK 
Molecular insights into atypical modes of beta-arrestin interaction with seven transmembrane receptors.
Maharana J  ,
Sano FK
,
Sano FK  ,
Sarma P
,
Sarma P  ,
Yadav MK
,
Yadav MK  ,
Duan L
,
Duan L  ,
Stepniewski TM
,
Stepniewski TM  ,
Chaturvedi M
,
Chaturvedi M  ,
Ranjan A
,
Ranjan A  ,
Singh V
,
Singh V  ,
Saha S
,
Saha S  ,
Mahajan G
,
Mahajan G  ,
Chami M
,
Chami M  ,
Shihoya W
,
Shihoya W  ,
Selent J
,
Selent J  ,
Chung KY
,
Chung KY  ,
Banerjee R
,
Banerjee R  ,
Nureki O
,
Nureki O  ,
Shukla AK
,
Shukla AK 
(2024) Science , 383 , 101 - 108
 ,
Sano FK
,
Sano FK  ,
Sarma P
,
Sarma P  ,
Yadav MK
,
Yadav MK  ,
Duan L
,
Duan L  ,
Stepniewski TM
,
Stepniewski TM  ,
Chaturvedi M
,
Chaturvedi M  ,
Ranjan A
,
Ranjan A  ,
Singh V
,
Singh V  ,
Saha S
,
Saha S  ,
Mahajan G
,
Mahajan G  ,
Chami M
,
Chami M  ,
Shihoya W
,
Shihoya W  ,
Selent J
,
Selent J  ,
Chung KY
,
Chung KY  ,
Banerjee R
,
Banerjee R  ,
Nureki O
,
Nureki O  ,
Shukla AK
,
Shukla AK 
(2024) Science , 383 , 101 - 108
Abstract:
β-arrestins (βarrs) are multifunctional proteins involved in signaling and regulation of seven transmembrane receptors (7TMRs), and their interaction is driven primarily by agonist-induced receptor activation and phosphorylation. Here, we present seven cryo-electron microscopy structures of βarrs either in the basal state, activated by the muscarinic receptor subtype 2 (M2R) through its third intracellular loop, or activated by the βarr-biased decoy D6 receptor (D6R). Combined with biochemical, cellular, and biophysical experiments, these structural snapshots allow the visualization of atypical engagement of βarrs with 7TMRs and also reveal a structural transition in the carboxyl terminus of βarr2 from a β strand to an α helix upon activation by D6R. Our study provides previously unanticipated molecular insights into the structural and functional diversity encoded in 7TMR-βarr complexes with direct implications for exploring novel therapeutic avenues.
β-arrestins (βarrs) are multifunctional proteins involved in signaling and regulation of seven transmembrane receptors (7TMRs), and their interaction is driven primarily by agonist-induced receptor activation and phosphorylation. Here, we present seven cryo-electron microscopy structures of βarrs either in the basal state, activated by the muscarinic receptor subtype 2 (M2R) through its third intracellular loop, or activated by the βarr-biased decoy D6 receptor (D6R). Combined with biochemical, cellular, and biophysical experiments, these structural snapshots allow the visualization of atypical engagement of βarrs with 7TMRs and also reveal a structural transition in the carboxyl terminus of βarr2 from a β strand to an α helix upon activation by D6R. Our study provides previously unanticipated molecular insights into the structural and functional diversity encoded in 7TMR-βarr complexes with direct implications for exploring novel therapeutic avenues.
