EMD-41730
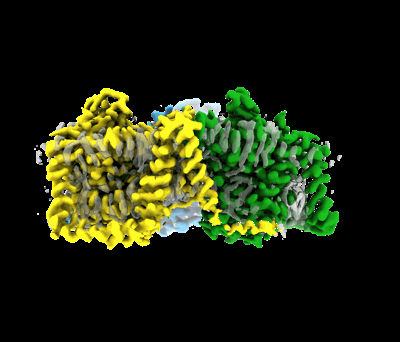
Cryo-EM structure of bovine concentrative nucleoside transporter 3 in complex with Ribavirin
EMD-41730
Single-particle2.54 Å
 Deposition: 26/08/2023
Deposition: 26/08/2023Map released: 13/03/2024
Last modified: 18/09/2024
Sample Organism:
Bos taurus
Sample: bCNT3 trimer
Fitted models: 8tz1 (Avg. Q-score: 0.596)
Deposition Authors: Wright NJ ,
Lee S-Y
,
Lee S-Y
Sample: bCNT3 trimer
Fitted models: 8tz1 (Avg. Q-score: 0.596)
Deposition Authors: Wright NJ
 ,
Lee S-Y
,
Lee S-Y
Antiviral drug recognition and elevator-type transport motions of CNT3.
Wright NJ  ,
Zhang F,
Suo Y
,
Zhang F,
Suo Y  ,
Kong L
,
Kong L  ,
Yin Y,
Fedor JG,
Sharma K
,
Yin Y,
Fedor JG,
Sharma K  ,
Borgnia MJ
,
Borgnia MJ  ,
Im W
,
Im W  ,
Lee SY
,
Lee SY 
(2024) Nat Chem Biol , 20 , 1144 - 1153
 ,
Zhang F,
Suo Y
,
Zhang F,
Suo Y  ,
Kong L
,
Kong L  ,
Yin Y,
Fedor JG,
Sharma K
,
Yin Y,
Fedor JG,
Sharma K  ,
Borgnia MJ
,
Borgnia MJ  ,
Im W
,
Im W  ,
Lee SY
,
Lee SY 
(2024) Nat Chem Biol , 20 , 1144 - 1153
Abstract:
Nucleoside analogs have broad clinical utility as antiviral drugs. Key to their systemic distribution and cellular entry are human nucleoside transporters. Here, we establish that the human concentrative nucleoside transporter 3 (CNT3) interacts with antiviral drugs used in the treatment of coronavirus infections. We report high-resolution single-particle cryo-electron microscopy structures of bovine CNT3 complexed with antiviral nucleosides N4-hydroxycytidine, PSI-6206, GS-441524 and ribavirin, all in inward-facing states. Notably, we found that the orally bioavailable antiviral molnupiravir arrests CNT3 in four distinct conformations, allowing us to capture cryo-electron microscopy structures of drug-loaded outward-facing and drug-loaded intermediate states. Our studies uncover the conformational trajectory of CNT3 during membrane transport of a nucleoside analog antiviral drug, yield new insights into the role of interactions between the transport and the scaffold domains in elevator-like domain movements during drug translocation, and provide insights into the design of nucleoside analog antiviral prodrugs with improved oral bioavailability.
Nucleoside analogs have broad clinical utility as antiviral drugs. Key to their systemic distribution and cellular entry are human nucleoside transporters. Here, we establish that the human concentrative nucleoside transporter 3 (CNT3) interacts with antiviral drugs used in the treatment of coronavirus infections. We report high-resolution single-particle cryo-electron microscopy structures of bovine CNT3 complexed with antiviral nucleosides N4-hydroxycytidine, PSI-6206, GS-441524 and ribavirin, all in inward-facing states. Notably, we found that the orally bioavailable antiviral molnupiravir arrests CNT3 in four distinct conformations, allowing us to capture cryo-electron microscopy structures of drug-loaded outward-facing and drug-loaded intermediate states. Our studies uncover the conformational trajectory of CNT3 during membrane transport of a nucleoside analog antiviral drug, yield new insights into the role of interactions between the transport and the scaffold domains in elevator-like domain movements during drug translocation, and provide insights into the design of nucleoside analog antiviral prodrugs with improved oral bioavailability.
