EMD-42003
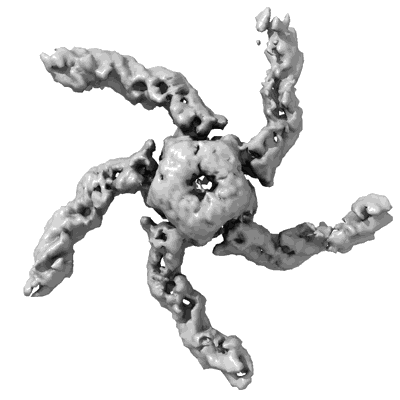
KCTD5/Cullin3/Gbeta1gamma2 Complex State B Body 2: RELION Multi-body Refinement of KCTD5(BTB)/Cullin3(NTD)
EMD-42003
Single-particle4.2 Å
 Deposition: 15/09/2023
Deposition: 15/09/2023Map released: 11/10/2023
Last modified: 01/05/2024
Sample Organism:
Homo sapiens
Sample: Complex of substrate adaptor KCTD5 with Cullin-3 and substrate Gbeta-gamma
Raw data: EMPIAR-11734
Deposition Authors: Nguyen DM ,
Narayanan N
,
Narayanan N  ,
Kuntz DA
,
Kuntz DA  ,
Prive GG
,
Prive GG 
Sample: Complex of substrate adaptor KCTD5 with Cullin-3 and substrate Gbeta-gamma
Raw data: EMPIAR-11734
Deposition Authors: Nguyen DM
 ,
Narayanan N
,
Narayanan N  ,
Kuntz DA
,
Kuntz DA  ,
Prive GG
,
Prive GG 
Structure and dynamics of a pentameric KCTD5/CUL3/G beta gamma E3 ubiquitin ligase complex.
Nguyen DM  ,
Rath DH
,
Rath DH  ,
Devost D
,
Devost D  ,
Petrin D,
Rizk R,
Ji AX,
Narayanan N
,
Petrin D,
Rizk R,
Ji AX,
Narayanan N  ,
Yong D
,
Yong D  ,
Zhai A,
Kuntz DA
,
Zhai A,
Kuntz DA  ,
Mian MUQ
,
Mian MUQ  ,
Pomroy NC
,
Pomroy NC  ,
Keszei AFA,
Benlekbir S,
Mazhab-Jafari MT
,
Keszei AFA,
Benlekbir S,
Mazhab-Jafari MT  ,
Rubinstein JL
,
Rubinstein JL  ,
Hebert TE
,
Hebert TE  ,
Prive GG
,
Prive GG 
(2024) PNAS , 121 , e2315018121 - e2315018121
 ,
Rath DH
,
Rath DH  ,
Devost D
,
Devost D  ,
Petrin D,
Rizk R,
Ji AX,
Narayanan N
,
Petrin D,
Rizk R,
Ji AX,
Narayanan N  ,
Yong D
,
Yong D  ,
Zhai A,
Kuntz DA
,
Zhai A,
Kuntz DA  ,
Mian MUQ
,
Mian MUQ  ,
Pomroy NC
,
Pomroy NC  ,
Keszei AFA,
Benlekbir S,
Mazhab-Jafari MT
,
Keszei AFA,
Benlekbir S,
Mazhab-Jafari MT  ,
Rubinstein JL
,
Rubinstein JL  ,
Hebert TE
,
Hebert TE  ,
Prive GG
,
Prive GG 
(2024) PNAS , 121 , e2315018121 - e2315018121
Abstract:
Heterotrimeric G proteins can be regulated by posttranslational modifications, including ubiquitylation. KCTD5, a pentameric substrate receptor protein consisting of an N-terminal BTB domain and a C-terminal domain, engages CUL3 to form the central scaffold of a cullin-RING E3 ligase complex (CRL3KCTD5) that ubiquitylates Gβγ and reduces Gβγ protein levels in cells. The cryo-EM structure of a 5:5:5 KCTD5/CUL3NTD/Gβ1γ2 assembly reveals a highly dynamic complex with rotations of over 60° between the KCTD5BTB/CUL3NTD and KCTD5CTD/Gβγ moieties of the structure. CRL3KCTD5 engages the E3 ligase ARIH1 to ubiquitylate Gβγ in an E3-E3 superassembly, and extension of the structure to include full-length CUL3 with RBX1 and an ARIH1~ubiquitin conjugate reveals that some conformational states position the ARIH1~ubiquitin thioester bond to within 10 Å of lysine-23 of Gβ and likely represent priming complexes. Most previously described CRL/substrate structures have consisted of monovalent complexes and have involved flexible peptide substrates. The structure of the KCTD5/CUL3NTD/Gβγ complex shows that the oligomerization of a substrate receptor can generate a polyvalent E3 ligase complex and that the internal dynamics of the substrate receptor can position a structured target for ubiquitylation in a CRL3 complex.
Heterotrimeric G proteins can be regulated by posttranslational modifications, including ubiquitylation. KCTD5, a pentameric substrate receptor protein consisting of an N-terminal BTB domain and a C-terminal domain, engages CUL3 to form the central scaffold of a cullin-RING E3 ligase complex (CRL3KCTD5) that ubiquitylates Gβγ and reduces Gβγ protein levels in cells. The cryo-EM structure of a 5:5:5 KCTD5/CUL3NTD/Gβ1γ2 assembly reveals a highly dynamic complex with rotations of over 60° between the KCTD5BTB/CUL3NTD and KCTD5CTD/Gβγ moieties of the structure. CRL3KCTD5 engages the E3 ligase ARIH1 to ubiquitylate Gβγ in an E3-E3 superassembly, and extension of the structure to include full-length CUL3 with RBX1 and an ARIH1~ubiquitin conjugate reveals that some conformational states position the ARIH1~ubiquitin thioester bond to within 10 Å of lysine-23 of Gβ and likely represent priming complexes. Most previously described CRL/substrate structures have consisted of monovalent complexes and have involved flexible peptide substrates. The structure of the KCTD5/CUL3NTD/Gβγ complex shows that the oligomerization of a substrate receptor can generate a polyvalent E3 ligase complex and that the internal dynamics of the substrate receptor can position a structured target for ubiquitylation in a CRL3 complex.
