EMD-4310
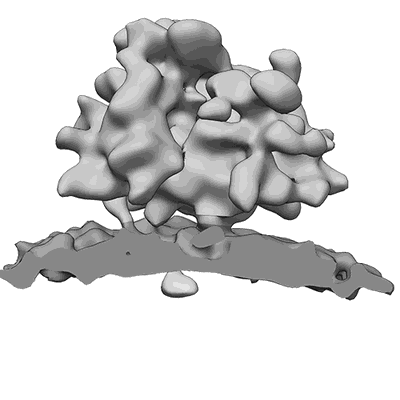
Subtomogram average of OST-free ribosome-translocon complexes from STT3A(-/-) HEK cells
EMD-4310
Subtomogram averaging30.0 Å
 Deposition: 22/02/2018
Deposition: 22/02/2018Map released: 21/03/2018
Last modified: 28/11/2018
Sample Organism:
Homo sapiens
Sample: 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic reticulum protein translocon
Deposition Authors: Pfeffer S, Foerster F
Sample: 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic reticulum protein translocon
Deposition Authors: Pfeffer S, Foerster F
Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum.
Braunger K  ,
Pfeffer S
,
Pfeffer S  ,
Shrimal S
,
Shrimal S  ,
Gilmore R
,
Gilmore R  ,
Berninghausen O
,
Berninghausen O  ,
Mandon EC
,
Mandon EC  ,
Becker T
,
Becker T  ,
Forster F
,
Forster F  ,
Beckmann R
,
Beckmann R 
(2018) Science , 360 , 215 - 219
 ,
Pfeffer S
,
Pfeffer S  ,
Shrimal S
,
Shrimal S  ,
Gilmore R
,
Gilmore R  ,
Berninghausen O
,
Berninghausen O  ,
Mandon EC
,
Mandon EC  ,
Becker T
,
Becker T  ,
Forster F
,
Forster F  ,
Beckmann R
,
Beckmann R 
(2018) Science , 360 , 215 - 219
Abstract:
Protein synthesis, transport, and N-glycosylation are coupled at the mammalian endoplasmic reticulum by complex formation of a ribosome, the Sec61 protein-conducting channel, and oligosaccharyltransferase (OST). Here we used different cryo-electron microscopy approaches to determine structures of native and solubilized ribosome-Sec61-OST complexes. A molecular model for the catalytic OST subunit STT3A (staurosporine and temperature sensitive 3A) revealed how it is integrated into the OST and how STT3-paralog specificity for translocon-associated OST is achieved. The OST subunit DC2 was placed at the interface between Sec61 and STT3A, where it acts as a versatile module for recruitment of STT3A-containing OST to the ribosome-Sec61 complex. This detailed structural view on the molecular architecture of the cotranslational machinery for N-glycosylation provides the basis for a mechanistic understanding of glycoprotein biogenesis at the endoplasmic reticulum.
Protein synthesis, transport, and N-glycosylation are coupled at the mammalian endoplasmic reticulum by complex formation of a ribosome, the Sec61 protein-conducting channel, and oligosaccharyltransferase (OST). Here we used different cryo-electron microscopy approaches to determine structures of native and solubilized ribosome-Sec61-OST complexes. A molecular model for the catalytic OST subunit STT3A (staurosporine and temperature sensitive 3A) revealed how it is integrated into the OST and how STT3-paralog specificity for translocon-associated OST is achieved. The OST subunit DC2 was placed at the interface between Sec61 and STT3A, where it acts as a versatile module for recruitment of STT3A-containing OST to the ribosome-Sec61 complex. This detailed structural view on the molecular architecture of the cotranslational machinery for N-glycosylation provides the basis for a mechanistic understanding of glycoprotein biogenesis at the endoplasmic reticulum.
