EMD-5525
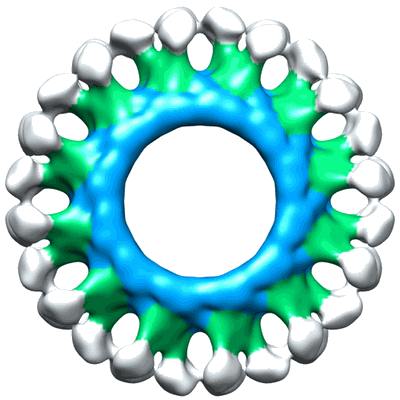
Electron cryo-microscopy of ABC BmrA in 12-fold symmetry rings
EMD-5525
Single-particle23.0 Å
 Deposition: 02/11/2012
Deposition: 02/11/2012Map released: 21/11/2012
Last modified: 14/05/2014
Sample Organism:
Bacillus subtilis
Sample: BmrA reconstituted at high density into lipid bilayer.
Deposition Authors: Fribourg PF, Chami M, Sorzano CO, Gubellini F ,
Marabini R
,
Marabini R  ,
Marco S,
Jault JM
,
Marco S,
Jault JM  ,
Levy D
,
Levy D 
Sample: BmrA reconstituted at high density into lipid bilayer.
Deposition Authors: Fribourg PF, Chami M, Sorzano CO, Gubellini F
 ,
Marabini R
,
Marabini R  ,
Marco S,
Jault JM
,
Marco S,
Jault JM  ,
Levy D
,
Levy D 
3D Cryo-Electron Reconstruction of BmrA, a Bacterial Multidrug ABC Transporter in an Inward-Facing Conformation and in a Lipidic Environment.
Fribourg PF,
Chami M,
Sorzano CO,
Gubellini F  ,
Marabini R
,
Marabini R  ,
Marco S,
Jault JM
,
Marco S,
Jault JM  ,
Levy D
,
Levy D 
(2014) J. Mol. Biol. , 426 , 2059 - 2069
 ,
Marabini R
,
Marabini R  ,
Marco S,
Jault JM
,
Marco S,
Jault JM  ,
Levy D
,
Levy D 
(2014) J. Mol. Biol. , 426 , 2059 - 2069
Abstract:
ABC (ATP-binding cassette) membrane exporters are efflux transporters of a wide diversity of molecule across the membrane at the expense of ATP. A key issue regarding their catalytic cycle is whether or not their nucleotide-binding domains (NBDs) are physically disengaged in the resting state. To settle this controversy, we obtained structural data on BmrA, a bacterial multidrug homodimeric ABC transporter, in a membrane-embedded state. BmrA in the apostate was reconstituted in lipid bilayers forming a mixture of ring-shaped structures of 24 or 39 homodimers. Three-dimensional models of the ring-shaped structures of 24 or 39 homodimers were calculated at 2.3 nm and 2.5 nm resolution from cryo-electron microscopy, respectively. In these structures, BmrA adopts an inward-facing open conformation similar to that found in mouse P-glycoprotein structure with the NBDs separated by 3 nm. Both lipidic leaflets delimiting the transmembrane domains of BmrA were clearly resolved. In planar membrane sheets, the NBDs were even more separated. BmrA in an ATP-bound conformation was determined from two-dimensional crystals grown in the presence of ATP and vanadate. A projection map calculated at 1.6 nm resolution shows an open outward-facing conformation. Overall, the data are consistent with a mechanism of drug transport involving large conformational changes of BmrA and show that a bacterial ABC exporter can adopt at least two open inward conformations in lipid membrane.
ABC (ATP-binding cassette) membrane exporters are efflux transporters of a wide diversity of molecule across the membrane at the expense of ATP. A key issue regarding their catalytic cycle is whether or not their nucleotide-binding domains (NBDs) are physically disengaged in the resting state. To settle this controversy, we obtained structural data on BmrA, a bacterial multidrug homodimeric ABC transporter, in a membrane-embedded state. BmrA in the apostate was reconstituted in lipid bilayers forming a mixture of ring-shaped structures of 24 or 39 homodimers. Three-dimensional models of the ring-shaped structures of 24 or 39 homodimers were calculated at 2.3 nm and 2.5 nm resolution from cryo-electron microscopy, respectively. In these structures, BmrA adopts an inward-facing open conformation similar to that found in mouse P-glycoprotein structure with the NBDs separated by 3 nm. Both lipidic leaflets delimiting the transmembrane domains of BmrA were clearly resolved. In planar membrane sheets, the NBDs were even more separated. BmrA in an ATP-bound conformation was determined from two-dimensional crystals grown in the presence of ATP and vanadate. A projection map calculated at 1.6 nm resolution shows an open outward-facing conformation. Overall, the data are consistent with a mechanism of drug transport involving large conformational changes of BmrA and show that a bacterial ABC exporter can adopt at least two open inward conformations in lipid membrane.
