EMD-6288
Electron cryo-microscopy of fatty acid synthase (FAS) from Rhodospiridium toruloides
EMD-6288
Single-particle7.8 Å
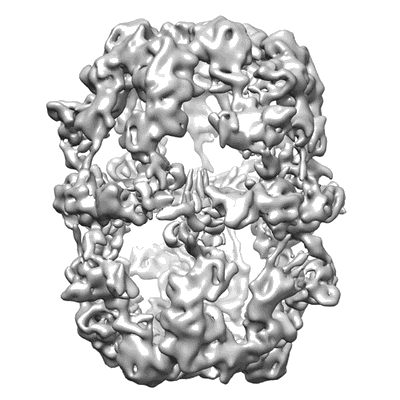 Deposition: 23/02/2015
Deposition: 23/02/2015Map released: 23/03/2016
Last modified: 23/03/2016
Sample Organism:
Rhodosporidium toruloides
Sample: Fatty acid synthase from Rhodospiridium toruloides
Deposition Authors: Fischer M, Rhinow D, Zhu Z ,
Mills DJ
,
Mills DJ  ,
Zhao ZK,
Vonck J
,
Zhao ZK,
Vonck J  ,
Grininger M
,
Grininger M 
Sample: Fatty acid synthase from Rhodospiridium toruloides
Deposition Authors: Fischer M, Rhinow D, Zhu Z
 ,
Mills DJ
,
Mills DJ  ,
Zhao ZK,
Vonck J
,
Zhao ZK,
Vonck J  ,
Grininger M
,
Grininger M 
Cryo-EM structure of fatty acid synthase (FAS) from Rhodosporidium toruloides provides insights into the evolutionary development of fungal FAS.
Fischer M,
Rhinow D,
Zhu Z  ,
Mills DJ
,
Mills DJ  ,
Zhao ZK,
Vonck J
,
Zhao ZK,
Vonck J  ,
Grininger M
,
Grininger M 
(2015) Protein Sci. , 24 , 987 - 995
 ,
Mills DJ
,
Mills DJ  ,
Zhao ZK,
Vonck J
,
Zhao ZK,
Vonck J  ,
Grininger M
,
Grininger M 
(2015) Protein Sci. , 24 , 987 - 995
Abstract:
Fungal fatty acid synthases Type I (FAS I) are up to 2.7 MDa large molecular machines composed of large multifunctional polypeptides. Half of the amino acids in fungal FAS I are involved in structural elements that are responsible for scaffolding the elaborate barrel-shaped architecture and turning fungal FAS I into highly efficient de novo producers of fatty acids. Rhodosporidium toruloides is an oleaginous fungal species and renowned for its robust conversion of carbohydrates into lipids to over 70% of its dry cell weight. Here, we use cryo-EM to determine a 7.8-Å reconstruction of its FAS I that reveals unexpected features; its novel form of splitting the multifunctional polypeptide chain into the two subunits α and β, and its duplicated ACP domains. We show that the specific distribution into α and β occurs by splitting at one of many possible sites that can be accepted by fungal FAS I. While, therefore, the specific distribution in α and β chains in R. toruloides FAS I is not correlated to increased protein activities, we also show that the duplication of ACP is an evolutionary late event and argue that duplication is beneficial for the lipid overproduction phenotype.
Fungal fatty acid synthases Type I (FAS I) are up to 2.7 MDa large molecular machines composed of large multifunctional polypeptides. Half of the amino acids in fungal FAS I are involved in structural elements that are responsible for scaffolding the elaborate barrel-shaped architecture and turning fungal FAS I into highly efficient de novo producers of fatty acids. Rhodosporidium toruloides is an oleaginous fungal species and renowned for its robust conversion of carbohydrates into lipids to over 70% of its dry cell weight. Here, we use cryo-EM to determine a 7.8-Å reconstruction of its FAS I that reveals unexpected features; its novel form of splitting the multifunctional polypeptide chain into the two subunits α and β, and its duplicated ACP domains. We show that the specific distribution into α and β occurs by splitting at one of many possible sites that can be accepted by fungal FAS I. While, therefore, the specific distribution in α and β chains in R. toruloides FAS I is not correlated to increased protein activities, we also show that the duplication of ACP is an evolutionary late event and argue that duplication is beneficial for the lipid overproduction phenotype.
