EMD-6527
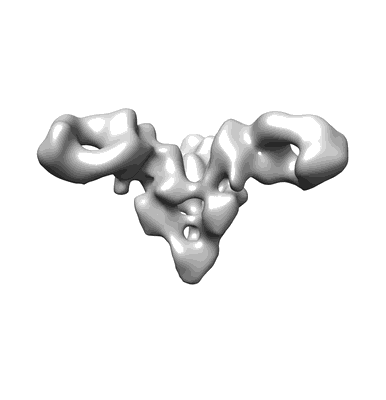
Negative stain EM reconstruction of Bundibugyo virus (BDBV) glycoprotein in complex with a human IgG1 Fab
EMD-6527
Single-particle17.0 Å
 Deposition: 20/11/2015
Deposition: 20/11/2015Map released: 06/04/2016
Last modified: 06/04/2016
Sample Organism:
Homo sapiens,
Ebola virus sp.
Sample: Fab fragment of BDBV335 human IgG1 monoclonal antibody to Bundibugyo virus in complex with mucin-deleted Bundibugyo virus GP
Deposition Authors: Flyak AI ,
Shen X,
Murin CD,
Turner HL,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N
,
Shen X,
Murin CD,
Turner HL,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N  ,
Branchizio A,
King H,
Brown L,
Davidson E,
Doranz BJ,
Slaughter JC
,
Branchizio A,
King H,
Brown L,
Davidson E,
Doranz BJ,
Slaughter JC  ,
Sapparapu G
,
Sapparapu G  ,
Klages C,
Ksiazek TG,
Saphire EO
,
Klages C,
Ksiazek TG,
Saphire EO  ,
Ward AB,
Bukreyev A,
Crowe JE
,
Ward AB,
Bukreyev A,
Crowe JE 
Sample: Fab fragment of BDBV335 human IgG1 monoclonal antibody to Bundibugyo virus in complex with mucin-deleted Bundibugyo virus GP
Deposition Authors: Flyak AI
 ,
Shen X,
Murin CD,
Turner HL,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N
,
Shen X,
Murin CD,
Turner HL,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N  ,
Branchizio A,
King H,
Brown L,
Davidson E,
Doranz BJ,
Slaughter JC
,
Branchizio A,
King H,
Brown L,
Davidson E,
Doranz BJ,
Slaughter JC  ,
Sapparapu G
,
Sapparapu G  ,
Klages C,
Ksiazek TG,
Saphire EO
,
Klages C,
Ksiazek TG,
Saphire EO  ,
Ward AB,
Bukreyev A,
Crowe JE
,
Ward AB,
Bukreyev A,
Crowe JE 
Cross-Reactive and Potent Neutralizing Antibody Responses in Human Survivors of Natural Ebolavirus Infection.
Flyak AI  ,
Shen X,
Murin CD,
Turner HL,
David JA
,
Shen X,
Murin CD,
Turner HL,
David JA  ,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N
,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N  ,
Branchizio A,
King H,
Brown L,
Bryan C,
Davidson E,
Doranz BJ,
Slaughter JC
,
Branchizio A,
King H,
Brown L,
Bryan C,
Davidson E,
Doranz BJ,
Slaughter JC  ,
Sapparapu G
,
Sapparapu G  ,
Klages C,
Ksiazek TG,
Saphire EO
,
Klages C,
Ksiazek TG,
Saphire EO  ,
Ward AB,
Bukreyev A,
Crowe JE
,
Ward AB,
Bukreyev A,
Crowe JE 
(2016) Cell , 164 , 392 - 405
 ,
Shen X,
Murin CD,
Turner HL,
David JA
,
Shen X,
Murin CD,
Turner HL,
David JA  ,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N
,
Fusco ML,
Lampley R,
Kose N,
Ilinykh PA,
Kuzmina N  ,
Branchizio A,
King H,
Brown L,
Bryan C,
Davidson E,
Doranz BJ,
Slaughter JC
,
Branchizio A,
King H,
Brown L,
Bryan C,
Davidson E,
Doranz BJ,
Slaughter JC  ,
Sapparapu G
,
Sapparapu G  ,
Klages C,
Ksiazek TG,
Saphire EO
,
Klages C,
Ksiazek TG,
Saphire EO  ,
Ward AB,
Bukreyev A,
Crowe JE
,
Ward AB,
Bukreyev A,
Crowe JE 
(2016) Cell , 164 , 392 - 405
Abstract:
Recent studies have suggested that antibody-mediated protection against the Ebolaviruses may be achievable, but little is known about whether or not antibodies can confer cross-reactive protection against viruses belonging to diverse Ebolavirus species, such as Ebola virus (EBOV), Sudan virus (SUDV), and Bundibugyo virus (BDBV). We isolated a large panel of human monoclonal antibodies (mAbs) against BDBV glycoprotein (GP) using peripheral blood B cells from survivors of the 2007 BDBV outbreak in Uganda. We determined that a large proportion of mAbs with potent neutralizing activity against BDBV bind to the glycan cap and recognize diverse epitopes within this major antigenic site. We identified several glycan cap-specific mAbs that neutralized multiple ebolaviruses, including SUDV, and a cross-reactive mAb that completely protected guinea pigs from the lethal challenge with heterologous EBOV. Our results provide a roadmap to develop a single antibody-based treatment effective against multiple Ebolavirus infections.
Recent studies have suggested that antibody-mediated protection against the Ebolaviruses may be achievable, but little is known about whether or not antibodies can confer cross-reactive protection against viruses belonging to diverse Ebolavirus species, such as Ebola virus (EBOV), Sudan virus (SUDV), and Bundibugyo virus (BDBV). We isolated a large panel of human monoclonal antibodies (mAbs) against BDBV glycoprotein (GP) using peripheral blood B cells from survivors of the 2007 BDBV outbreak in Uganda. We determined that a large proportion of mAbs with potent neutralizing activity against BDBV bind to the glycan cap and recognize diverse epitopes within this major antigenic site. We identified several glycan cap-specific mAbs that neutralized multiple ebolaviruses, including SUDV, and a cross-reactive mAb that completely protected guinea pigs from the lethal challenge with heterologous EBOV. Our results provide a roadmap to develop a single antibody-based treatment effective against multiple Ebolavirus infections.
