EMD-11964
Puumala virus-like particle glycoprotein spike in complex with fab fragment P-4G2.
EMD-11964
Subtomogram averaging13.4 Å
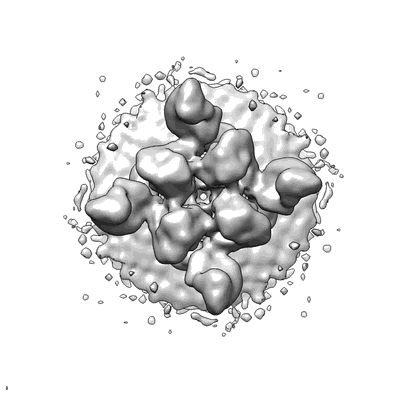 Deposition: 19/11/2020
Deposition: 19/11/2020Map released: 16/12/2020
Last modified: 13/11/2024
Sample Organism:
Myodes glareolus,
Puumala orthohantavirus,
Puumala virus - Sotkamo
Sample: Puumala virus - Sotkamo
Fitted models: 7b09 (Avg. Q-score: 0.077)
Deposition Authors: Rissanen I, Stass R, Huiskonen JT ,
Bowden TA
,
Bowden TA 
Sample: Puumala virus - Sotkamo
Fitted models: 7b09 (Avg. Q-score: 0.077)
Deposition Authors: Rissanen I, Stass R, Huiskonen JT
 ,
Bowden TA
,
Bowden TA 
Molecular rationale for antibody-mediated targeting of the hantavirus fusion glycoprotein.
Rissanen I,
Stass R,
Krumm SA,
Seow J,
Hulswit RJ  ,
Paesen GC,
Hepojoki J
,
Paesen GC,
Hepojoki J  ,
Vapalahti O
,
Vapalahti O  ,
Lundkvist A,
Reynard O
,
Lundkvist A,
Reynard O  ,
Volchkov V,
Doores KJ,
Huiskonen JT
,
Volchkov V,
Doores KJ,
Huiskonen JT  ,
Bowden TA
,
Bowden TA 
(2020) eLife , 9
 ,
Paesen GC,
Hepojoki J
,
Paesen GC,
Hepojoki J  ,
Vapalahti O
,
Vapalahti O  ,
Lundkvist A,
Reynard O
,
Lundkvist A,
Reynard O  ,
Volchkov V,
Doores KJ,
Huiskonen JT
,
Volchkov V,
Doores KJ,
Huiskonen JT  ,
Bowden TA
,
Bowden TA 
(2020) eLife , 9
Abstract:
The intricate lattice of Gn and Gc glycoprotein spike complexes on the hantavirus envelope facilitates host-cell entry and is the primary target of the neutralizing antibody-mediated immune response. Through study of a neutralizing monoclonal antibody termed mAb P-4G2, which neutralizes the zoonotic pathogen Puumala virus (PUUV), we provide a molecular-level basis for antibody-mediated targeting of the hantaviral glycoprotein lattice. Crystallographic analysis demonstrates that P-4G2 binds to a multi-domain site on PUUV Gc and may preclude fusogenic rearrangements of the glycoprotein that are required for host-cell entry. Furthermore, cryo-electron microscopy of PUUV-like particles in the presence of P-4G2 reveals a lattice-independent configuration of the Gc, demonstrating that P-4G2 perturbs the (Gn-Gc)4 lattice. This work provides a structure-based blueprint for rationalizing antibody-mediated targeting of hantaviruses.
The intricate lattice of Gn and Gc glycoprotein spike complexes on the hantavirus envelope facilitates host-cell entry and is the primary target of the neutralizing antibody-mediated immune response. Through study of a neutralizing monoclonal antibody termed mAb P-4G2, which neutralizes the zoonotic pathogen Puumala virus (PUUV), we provide a molecular-level basis for antibody-mediated targeting of the hantaviral glycoprotein lattice. Crystallographic analysis demonstrates that P-4G2 binds to a multi-domain site on PUUV Gc and may preclude fusogenic rearrangements of the glycoprotein that are required for host-cell entry. Furthermore, cryo-electron microscopy of PUUV-like particles in the presence of P-4G2 reveals a lattice-independent configuration of the Gc, demonstrating that P-4G2 perturbs the (Gn-Gc)4 lattice. This work provides a structure-based blueprint for rationalizing antibody-mediated targeting of hantaviruses.
