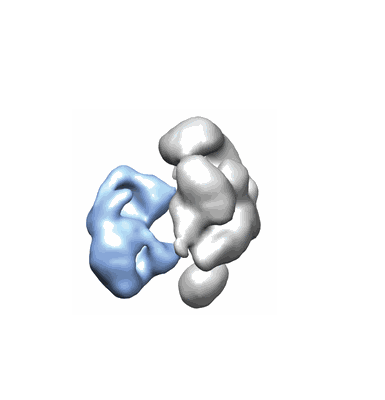
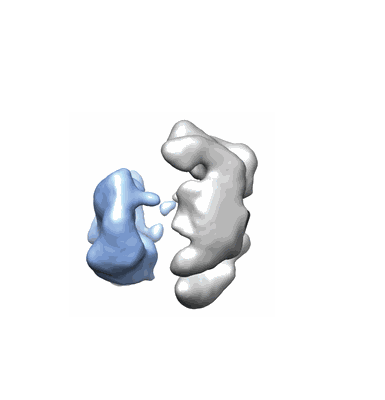
Drosophila Dicer-2 bound to blunt dsRNA
Resolution: 6.8 Å
EM Method: Single-particle
Fitted PDBs: 6bu9
Q-score: 0.193
Sinha NK, Iwasa J, Shen PS, Bass BL
Science (2018) 359 pp. 329-334 [ DOI: doi:10.1126/science.aaq0921 Pubmed: 29269422 ]
- Drosophila dicer-2 bound to blunt dsrna (Complex from Drosophila melanogaster)
- Rna (5'-r(*ap*cp*up*ap*cp*up*ap*up*ap*cp*ap*ap*cp*cp*up*ap*c)-3') (16 kDa, RNA from unidentified)
- Dicer-2, isoform a (198 kDa, Protein from Drosophila melanogaster)
- Rna (5'-r(*gp*gp*ap*gp*gp*up*ap*gp*up*ap*gp*gp*up*up*gp*up*ap*up*ap*gp*up*ap*gp*u)-3') (16 kDa, RNA from unidentified)
BG505 SOSIP.664 HIV-1 Env trimer in complex with anti-HIV fusion peptide targeting N123-VRC34.01 Fab
Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, Ozorowski G, Soto C, Taft JD, Bailer RT, Cale EM, Chen L, Choi CW, Chuang GY, Doria-Rose NA, Druz A, Georgiev IS, Gorman J, Huang J, Joyce MG, Louder MK, Ma X, McKee K, O'Dell S, Pancera M, Yang Y, Blanchard SC, Mothes W, Burton DR, Koff WC, Connors M, Ward AB, Kwong PD, Mascola JR
Science (2016) 352 pp. 828-833 [ Pubmed: 27174988 DOI: doi:10.1126/science.aae0474 ]
A Unique Human Mycoplasma Protein that Generically Blocks Antigen-Antibody Union
Grover RK, Zhu X, Nieusma T, Jones T, Boero I, MacLeod AS, Mark A, Niessen S, Kim HJ, Kong L, Assad-Garcia N, Kwon K, Chesi M, Smider VV, Salomon DR, Jelinek DF, Kyle RA, Pyles RB, Glass JI, Ward AB, Wilson IA, Lerner RA
Science (2014) 343 pp. 656-661 [ DOI: doi:10.1126/science.1246135 Pubmed: 24503852 ]
- Mab b12 fab (Protein from Homo sapiens)
- Fab of human mab b12 in complex with trypsinized mg281 (100 kDa, Sample)
- Mg281t (50 kDa, Protein from Mycoplasma genitalium)
Structures of Cas9 endonucleases reveal RNA-mediated conformational activation
Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA
Science (2014) 343 pp. 1247997- [ DOI: doi:10.1126/science.1247997 Pubmed: 24505130 ]
- Apo-cas9 (160 kDa, Sample)
- Crispr-associated endonuclease cas9 (159 kDa, Protein from Streptococcus pyogenes)
Structures of Cas9 endonucleases reveal RNA-mediated conformational activation
Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA
Science (2014) 343 pp. 1247997- [ DOI: doi:10.1126/science.1247997 Pubmed: 24505130 ]
- Crispr rna (14 kDa, RNA from Streptococcus pyogenes)
- Cas9 loaded with crrna:tracrrna (196 kDa, Sample)
- Trans-activating crispr rna (24 kDa, RNA from Streptococcus pyogenes)
- Crispr-associated endonuclease cas9 (159 kDa, Protein from Streptococcus pyogenes)
Structures of Cas9 endonucleases reveal RNA-mediated conformational activation
Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA
Science (2014) 343 pp. 1247997- [ DOI: doi:10.1126/science.1247997 Pubmed: 24505130 ]
- Crispr-associated endonuclease cas9 (159 kDa, Protein from Streptococcus pyogenes)
- Crispr rna (14 kDa, RNA from Streptococcus pyogenes)
- 55 bp dna duplex non-target strand (17 kDa, DNA from Enterobacteria phage lambda)
- Cas9 loaded with crrna:tracrrna and bound to target dna duplex (230 kDa, Sample)
- 55 bp dna duplex target strand (17 kDa, DNA from Enterobacteria phage lambda)
- Trans-activating crispr rna (24 kDa, RNA from Streptococcus pyogenes)
Organization of the Influenza Virus Replication Machinery
Resolution: 21.0 Å
EM Method: Helical reconstruction
Fitted PDBs: 2ymn
Q-score: 0.046
Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA
Science (2012) 338 pp. 1631-1634 [ DOI: doi:10.1126/science.1227270 Pubmed: 23180774 ]
Organization of the Influenza Virus Replication Machinery
Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA
Science (2012) 338 pp. 1631-1634 [ DOI: doi:10.1126/science.1227270 Pubmed: 23180774 ]
Organization of the Influenza Virus Replication Machinery
Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA
Science (2012) 338 pp. 1631-1634 [ DOI: doi:10.1126/science.1227270 Pubmed: 23180774 ]
Organization of the Influenza Virus Replication Machinery
Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA
Science (2012) 338 pp. 1631-1634 [ DOI: doi:10.1126/science.1227270 Pubmed: 23180774 ]