
The images for September in our 2020 PDBe calendar are inspired by a protein that flows through our veins and arteries, transporting the the most vital molecule we need to survive and thrive.
Haemoglobin
Haemoglobin - from the ancient Greek ‘haematin’, meaning blood, and the latin ‘globin’ for sphere - is literally the ‘blood protein’. It is the molecule responsible for transporting oxygen from the lungs to the rest of your body through the vast transport network of your blood vessels. It is vital for ensuring that oxygen is consistently provided to all cells for respiration, allowing energy to be generated for movement, growth and repair. Haemoglobin and oxygen transport is so vital that it is one of the most abundant proteins found in the Human body. It is packed into the red blood cells that transport it, with around 250 million molecules in each cell. That equates to over half a kilogram of haemoglobin in the average adult Human!
Haem
Though haemoglobin is a protein, its function is dependent on another molecule that binds to the protein, called haem. Haem is an iron-containing porphyrin molecule; a wide and flat compound with an iron ion in the centre. The nature of the chemistry of a haem molecule means that a conjugated system of bonding is created, forming a large, stable network. Molecules such as this are known as pigments, and are able to absorb energy from photons of light, emitting this back at specific wavelengths to produce what we observe as colour. The specific combination of conjugation and the presence of the iron give haem a red colour, hence why our blood is red. Oxygenated haemoglobin (oxyhaemoglobin) is bright red, however deoxyhaemoglobin is instead a darker red, though this can appear blue through your skin.
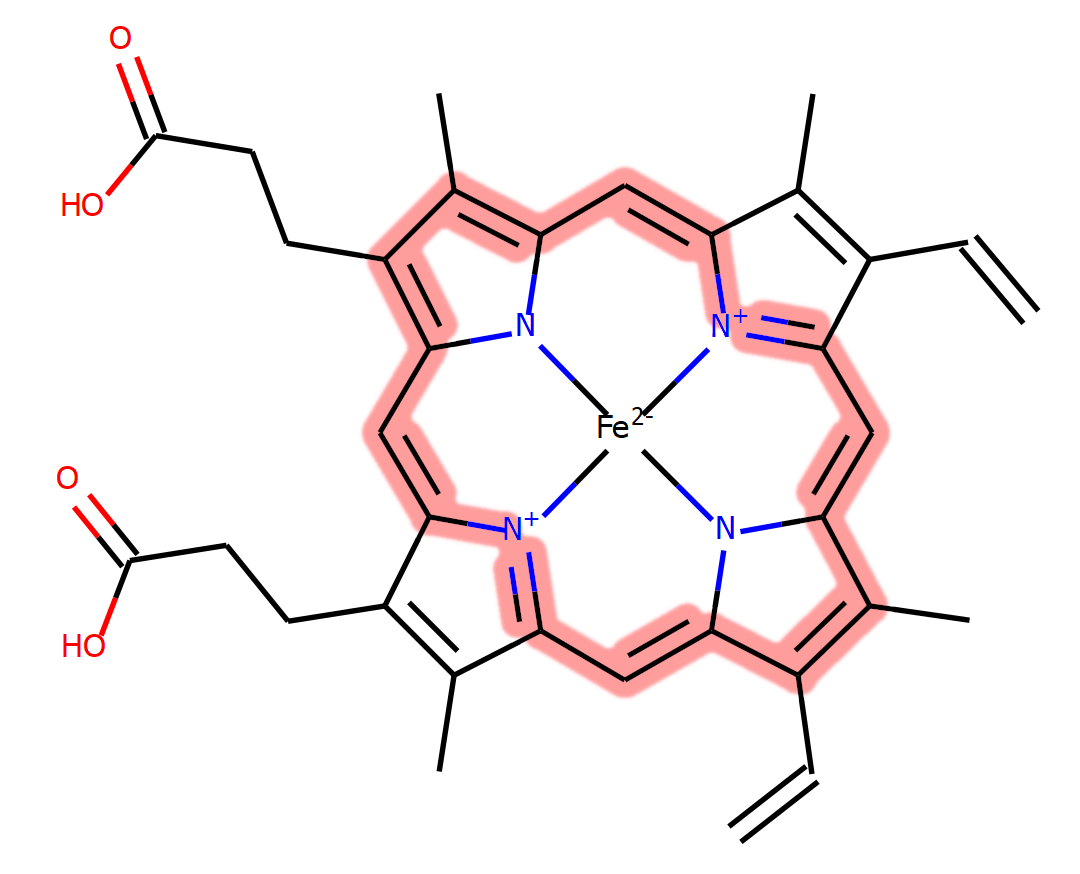
A chemical diagram of haem, with carbons shown as lines and hydrogens not displayed. The conjugated bond network is highlighted in pink. You can view information about haem (HEM) in the PDB at our PDBe chemistry pages.
Binding oxygen
The haem molecule is crucial for the binding of oxygen. Each haemoglobin molecule has 4 copies of the pigment, therefore can carry up to 4 oxygen molecules. It is the haem that ‘picks up’ the oxygen molecules, with the iron at the centre interacting with them directly. The flat nature of the haem molecule means that there is space either side of the iron for it to make additional interactions. When bound to haemoglobin, the iron already interacts on one side with the protein, however this leaves the other side available to bind molecular oxygen.
But haemoglobin is not only an oxygen carrier, it can also bind carbon dioxide (CO2), though it does so at a separate binding site. Once oxyhaemoglobin arrives at areas that need oxygen and contain high levels of CO2, it drops off the oxygen and picks up CO2. The opposite process then takes place at the lungs where the oxygen concentration is high, allowing the haemoglobin to reoxygenate and continue to supply the body with oxygen.
The structure of haemoglobin
Haemoglobin was one of the first protein structures solved, earning Max Perutz the 1962 Nobel Prize in Chemistry, along with his close collaborator John Kendrew. Kendrew had solved the structure of myoglobin, a related structure of haemoglobin and used to transport oxygen throughout muscle tissues. Haemoglobin is composed of four protein subunits, 2 alpha chains and 2 beta chains, while myoglobin consists of only a single subunit.
The alpha and beta subunits of haemoglobin vary in their sequence, however have roughly similar 3D structures. These subunits are arranged so that each alpha subunit makes contact with each beta subunit, yet not with the other alpha subunit. Each of these contains a haem group, leading to the four oxygen binding sites in the overall haemoglobin structure. The importance of the haem and oxygen binding site is highlighted in the fact that this is the region with the most highly conserved amino acids.
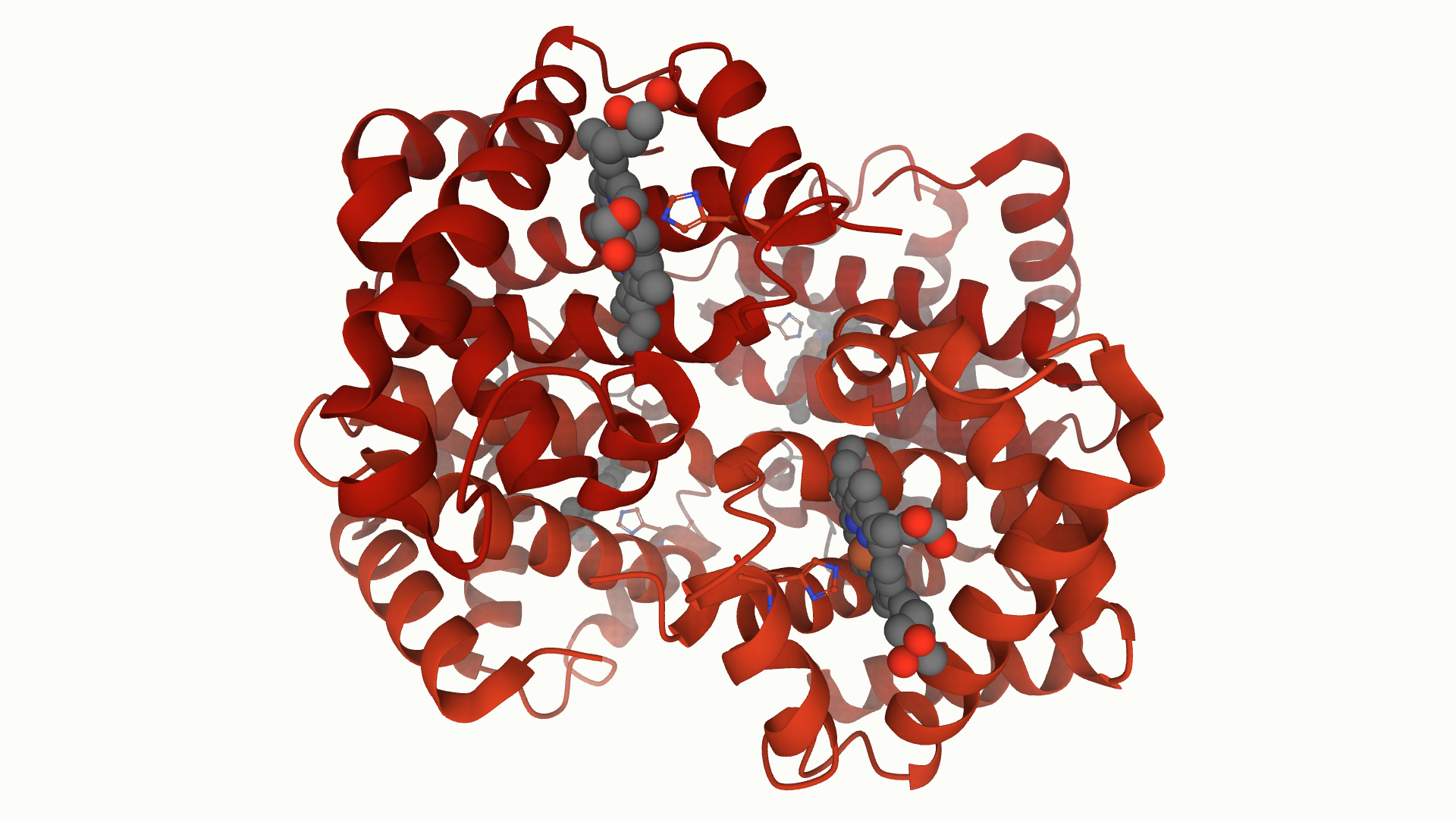
The haemoglobin protein in PDB entry 4hhb, displayed in Mol*. The alpha and beta subunits are displayed in cartoon format and are dark and bright red, respectively. Each haem molecule is displayed in sphere representation with standard atomic colouring, with the coordinating histidine also displayed in ball and sticks.
The oxygen binding site
Though the haem molecule directly interacts with the molecular oxygen, it is the surrounding amino acids from the haemoglobin protein that fine tune the binding and release of oxygen. The haem sits deep within haemoglobin with the iron coordinated on one side by a conserved histidine residue from the protein. Another histidine residue sits on the other side of the haem but does not interact directly with the iron, instead it helps to weaken the binding of the oxygen and allow its release when required.
Despite haem’s planar nature, it does not sit flat in the binding site when oxygen is not bound. The lack of coordination on one side of the haem causes a ‘domed’ conformation of the molecule, with the iron drawn towards the coordinating histidine residue. When oxygen binds to each subunit, the interactions across the plane of the haem become more balanced, causing it to flatten. This causes a number of nearby residues to get ‘pushed’ away from this site, leading to significant conformational changes in the protein.
T & R structures and cooperativity
There are therefore two main structural conformations of the haemoglobin subunits, the deoxy (T) structure and the oxy (R) structure. These two conformations are characterised by a rotation of the interface between an alpha and beta subunit, with the T structure containing a number of salt bridges that are absent in the R structure. It is the flattening of the haem molecule that triggers this large conformational change in the protein, leading to rearrangement of amino acids and breaking of the salt bridges.
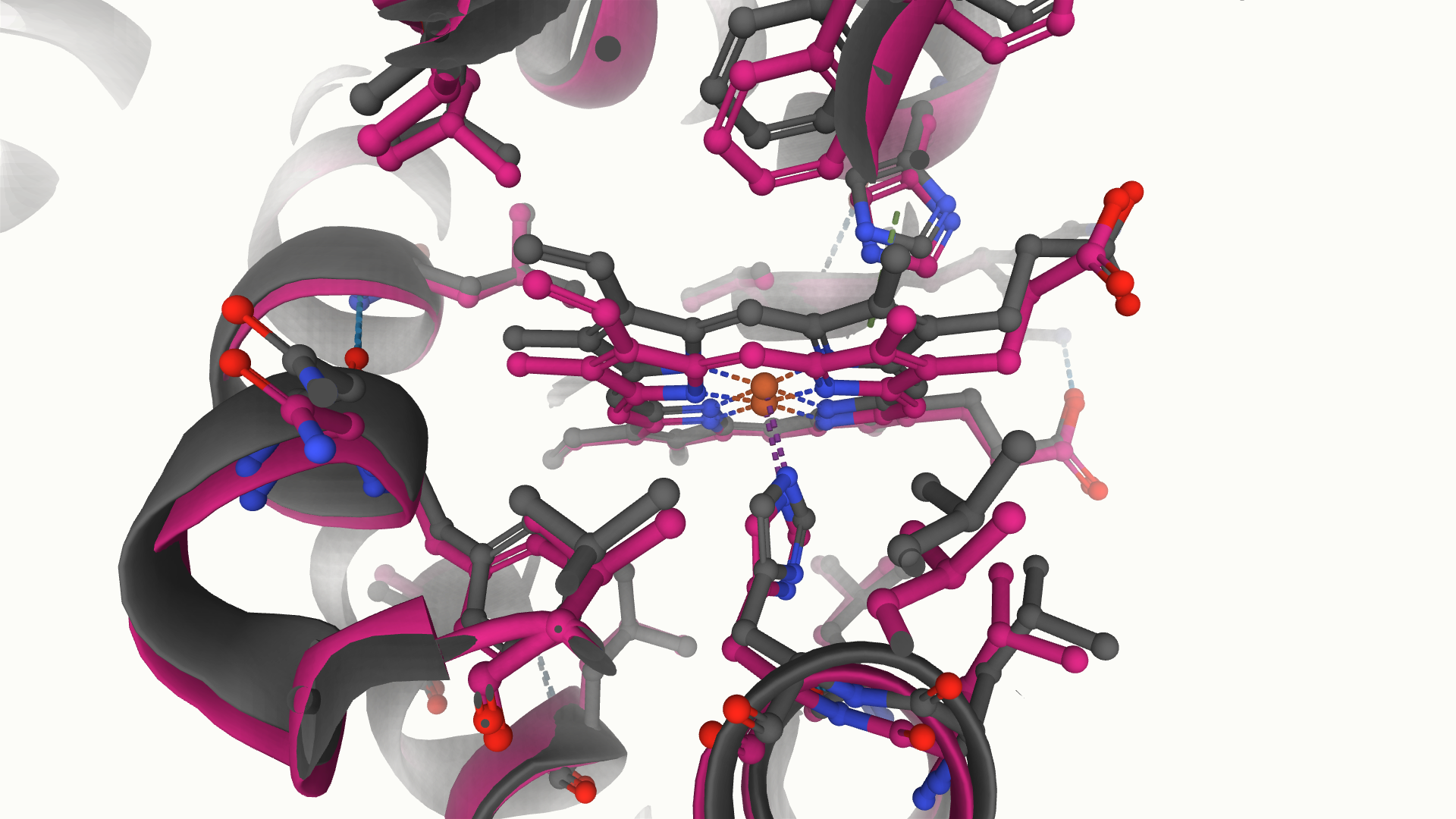
Two states of haemoglobin, mirroring the transition between the T and R structures, solved by cryoEM (PDB entries 6nbc and 6nbd). In the grey structure (6nbd), the haem molecule adopts a more domed conformation, while in the pink structure (6nbc) the haem is flattened, leading to significant rearrangement of surrounding amino acids.
The removal of these salt bridges after the binding of a single oxygen molecule is very useful, as these otherwise restrict the access to the oxygen binding site. As each successive oxygen binds and the structure moves towards the R structure, the number of salt bridges to be broken reduces, therefore promoting the binding of the next oxygen. This phenomenon is called cooperativity and ensures that the maximum number of oxygen molecules is picked up per haemoglobin protein. This is a remarkably fine-tuned process and massively beneficial for oxygen transport.
Haemoglobin in disease
The importance of haemoglobin in providing oxygen for respiration, one of the most key processes in living organisms, means that it is also associated with a number of diseases. One of the main haemoglobin-related diseases is anaemia, where there is a large reduction in the number of red blood cells and haemoglobin in the blood. This is often caused by a lack of iron, meaning not enough is available to make the vast quantities of haem required in the body. The subsequent reduction in oxygen transport means that anaemia sufferers experience tiredness and shortness of breath.
Another variation of this disease is sickle cell anaemia, again a disease characterised by a lack of haemoglobin for oxygen absorption and transport. This, however, is a genetic disorder, caused by a single mutation on the surface of the haemoglobin protein. In low oxygen, the mutated T structure of sickle-cell haemoglobin exposes a hydrophobic patch that causes association of haemoglobin molecules into large, fibrous precipitates. These lead to a distortion of the red blood cells, forming into a sickle-like curved shape rather than the usual smooth, doughnut shape. These cells become much more fragile and often cannot return to their original shape, meaning a reduction in haemoglobin and oxygen transport.
The artworks
The artworks at the top of this piece are inspired by haemoglobin and the red blood cells in which it is found. The print on the left was created by Daisy Barton Gilheany from Impington Village College and is an illustration of the 3D structure of haemoglobin. The sculpture on the right was created by Rebecca Koch, also from Impington, and depicts a collection of red blood cells and viruses within a blood vessel.
David Armstrong


