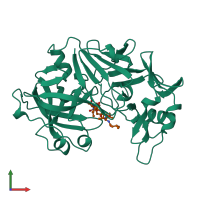
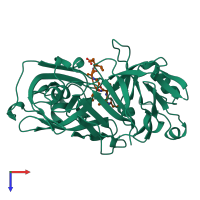
Function and Biology Details
Reaction catalysed:
Preferential cleavage: hydrophobic, preferably aromatic, residues in P1 and P1' positions. Cleaves 1-Phe-|-Val-2, 4-Gln-|-His-5, 13-Glu-|-Ala-14, 14-Ala-|-Leu-15, 15-Leu-|-Tyr-16, 16-Tyr-|-Leu-17, 23-Gly-|-Phe-24, 24-Phe-|-Phe-25 and 25-Phe-|-Tyr-26 bonds in the B chain of insulin.
Biochemical function:
Biological process:
Cellular component:
- not assigned
Sequence domains:
Structure domain:
Structure analysis Details
Assembly composition:
hetero dimer (preferred)
Assembly name:
Pepsin A-4 and peptide (preferred)
PDBe Complex ID:
PDB-CPX-534078 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
Spacegroup:
P212121
Expression system: Not provided