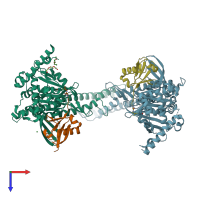
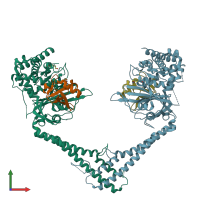Function and Biology Details
Reaction catalysed:
Thiol-dependent hydrolysis of ester, thioester, amide, peptide and isopeptide bonds formed by the C-terminal Gly of ubiquitin (a 76-residue protein attached to proteins as an intracellular targeting signal).
Biochemical function:
Biological process:
Cellular component:
- not assigned
Structure analysis Details
Assembly composition:
hetero tetramer (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-533796 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):