
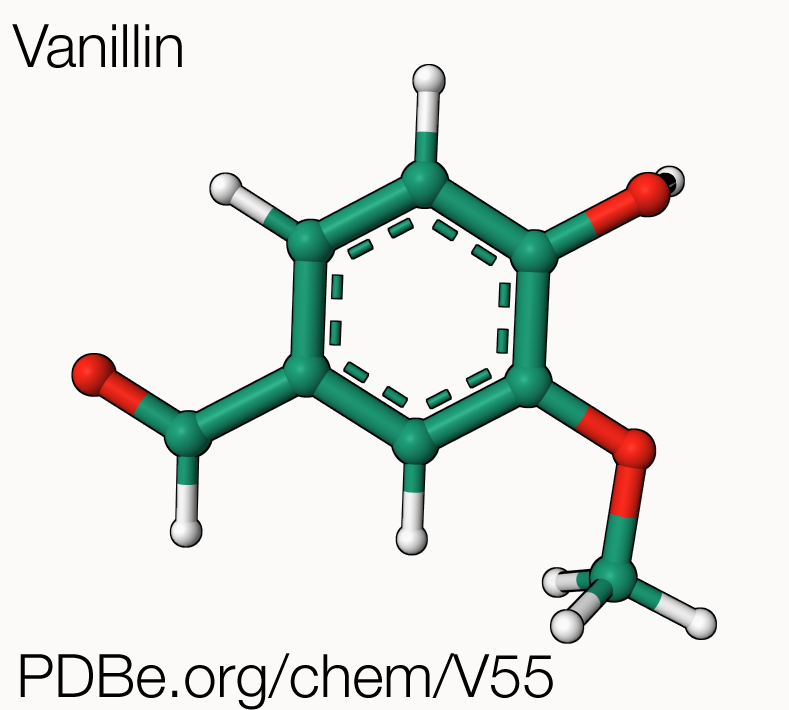
Vanilla and vanillin
Natural vanillin, the chemical compound behind the “vanilla” flavour, is extracted from the seed pods of Vanilla planifolia, a vining orchid native to Mexico, but now grown in tropical areas around the globe. Today, the term "vanilla" is synonymous with "common", but for many centuries, vanilla was a rare and highly sought-after flavour, enjoyed only by wealthy Europeans. This all changed in 1841 thanks to Edmond Albius, an enslaved worker in the French colony of Réunion, who discovered how to hand-pollinate vanilla orchids to produce vanilla beans, greatly increasing production and allowing the cultivation of vanilla to expand to Madagascar. Natural vanilla grown in different locations, such as Madagascar, Mexico, or Tahiti, can have distinct taste and strength profiles, similar to how different wines taste and feel differently. Madagascan vanilla, also known as Bourbon vanilla, is particularly prized for its rum-like flavour and sweet aroma. Nowadays, most of the world's natural vanilla is grown on smallholder farms in Madagascar, where it is hand-pollinated and cured in a traditional manner. Production of vanillin from the orchids is laborious: 500 kg of vanilla pods yield only 1 kg of vanillin! The demand for vanilla has consistently exceeded supply, leading to the synthesis of vanillin.
Natural vanilla extracted from the vanilla beans is made up of a blend of several hundred different compounds, including vanillin. The artificial vanilla flavouring used in sweet foods like ice cream and chocolate often consists of pure vanillin, and makes up for up to 75% of the vanillin market. Most of the vanillin available today is not extracted from vanilla beans but is instead produced synthetically through various methods such as the use of guaiacol, pine bark, clove oil, rice bran, or lignin. In fact, about 85% of the world's annual vanillin production (around 18,000 metric tons) has been synthesized from the petrochemical precursor guaiacol, with most of the rest coming from lignin manufactured by Norwegian companies.
Consumer demand for natural foods has led to a resurgence of interest in traditional vanilla beans, and food companies are working to find additional sources of natural vanillin and improve the quality and quantity of bean-derived vanilla. However, the production of natural vanilla is small and has been declining in recent years, and food companies face challenges such as rising costs, reformulation difficulties, and complicated labelling laws in their efforts to use natural vanilla.
Biosynthesis of vanillin
Food companies that choose not to use synthetic vanillin can instead use natural vanillin from alternative sources, for example through the fermentation of ferulic acid, a by-product of rice bran oil. In the pods of the vanilla orchid, this reaction is mediated by a single enzyme called vanillin synthase. In other environments or organisms, such as in Pseudomonas fluorescens, a gram-negative bacterium found in a soil, water, and plants, a similar reaction has been demonstrated for the bioconversion of the Coenzyme A-thioester of ferulic acid to vanillin.
The bioconversion of ferulic acid into vanillin happens as a sequential two-stages process: an initial hydration addition reaction, followed by a retro-aldol elimination reaction (Fig. 2). In Pseudomonas fluorescens, the first step being catalysed by the enzyme called hydroxycinnamate-CoA ligase-synthase enzyme (HCLS), the second step by the enzyme Hydroxycinnamoyl-CoA Hydratase-Lyase (HCHL).
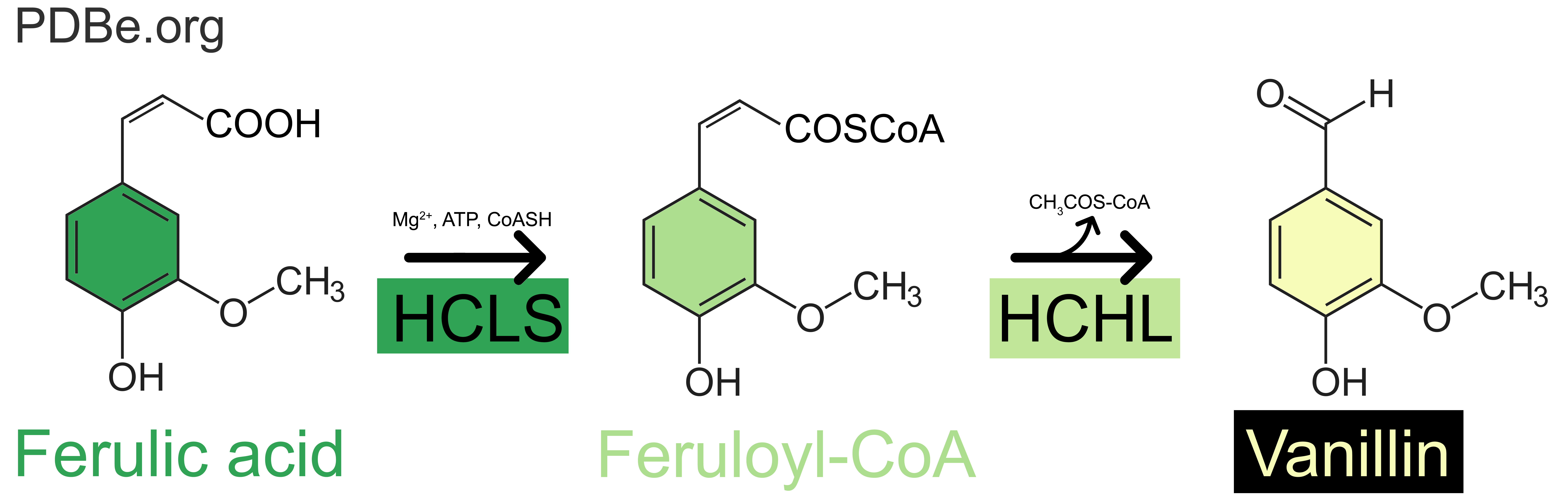
Interestingly, there is no sequence similarity between V. planifolia’s vanillin synthase and P. fluorescens HCHL, suggesting that, although the end product of the reaction they catalyse is the same, they likely evolved separately.
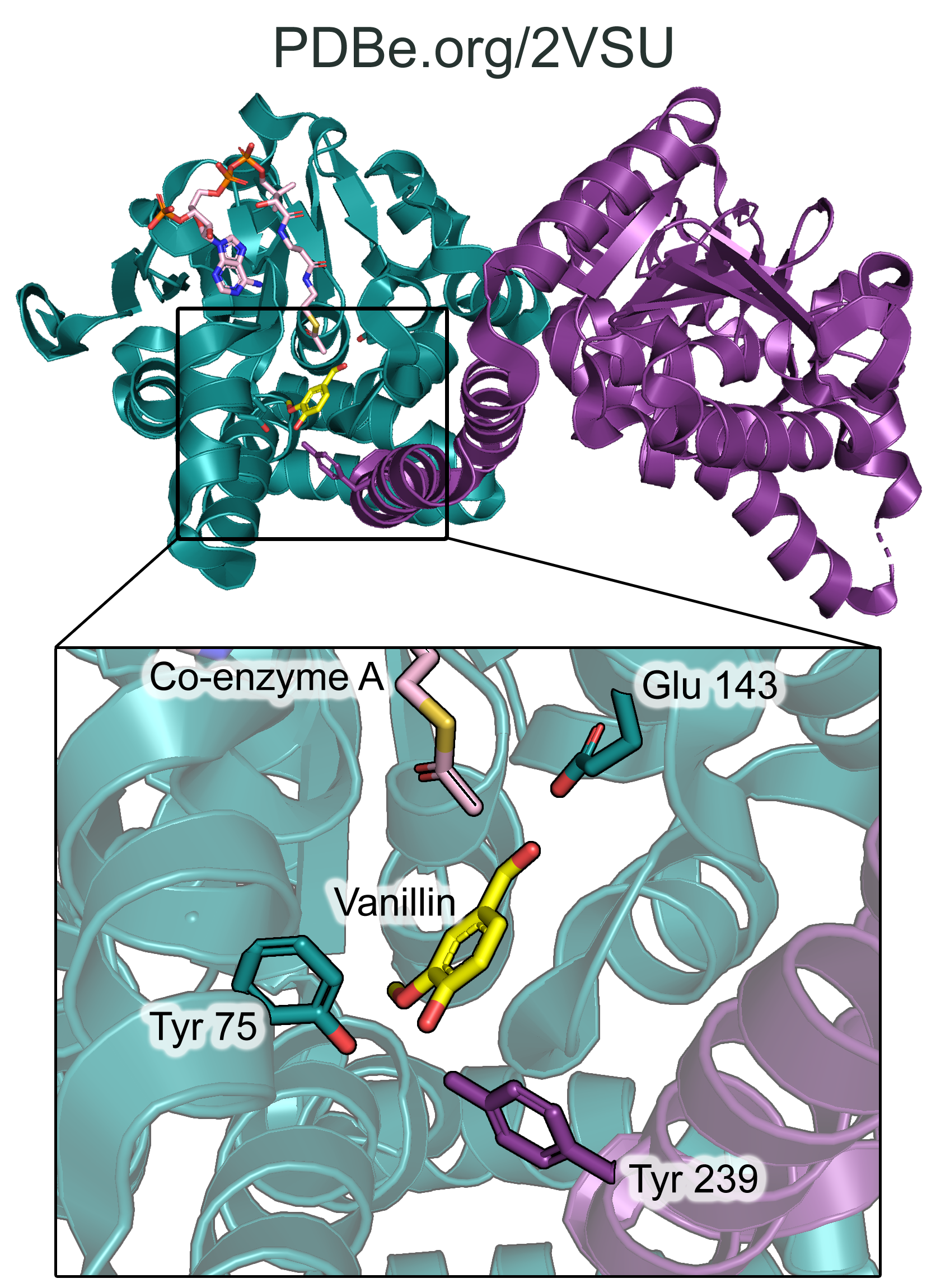
The structure of HCHL was determined using X-ray crystallography, and is available in the PDB under the ID 2VSU. The structure shows trimers of dimers of HCHL co-crystallized with feruloyl-CoA and vanillin (observed in chain D). Kinetic analysis of active-site residues identified using the crystal structure of HCHL revealed that Glu-143 and Tyr-75 are essential for activity. The active site also requires cooperativity from a neighbouring subunit in the dimer: the hydroxyl group of Tyr-239 from a neighbouring subunit is in close proximity to Tyr-75, and they bind together the phenolic hydroxyl of vanillin (Fig. 3). The mutation of Tyr-239 to Phe also greatly increased the Km for ferulic acid.
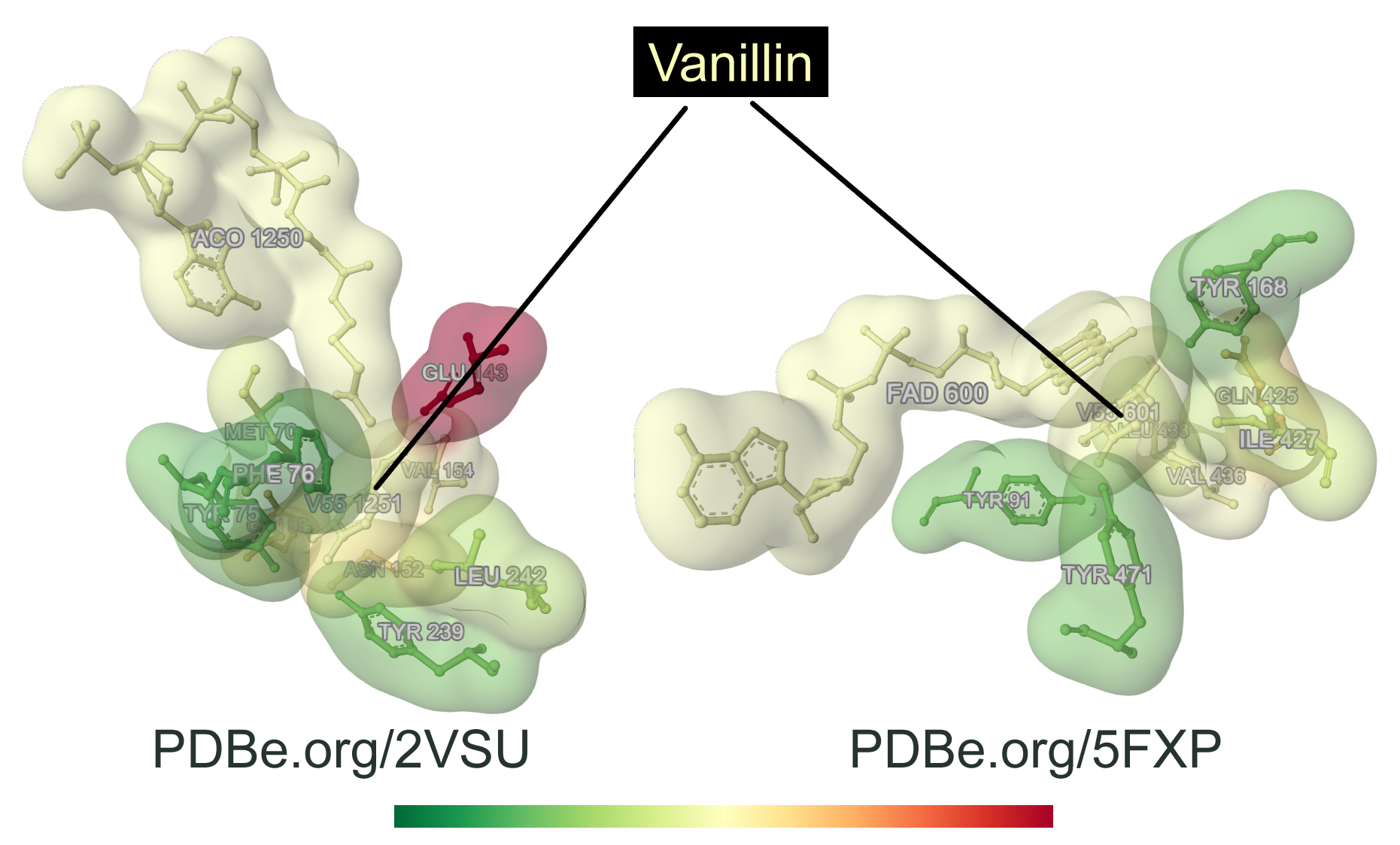
When looking at other proteins capable of binding vanillin and vanillin-related products, it appears that the active-site residues of HCHL bound to vanillin display a similar environment with those of eugenol oxidase from Rhodococcus jostii (PDB 5FXP), that also recognizes p-hydroxylated aromatic substrates related to vanillin, both active sites being highly hydrophobic (Fig. 4). Those two enzymes are structurally unrelated, showing an interesting convergence of the molecular determinants of vanillin-related ligand recognition.
So… why do old books smell so nice?
The distinctive "old book smell" can be accounted for by several different volatile organic compounds, one of which being vanillin!
HCHL can not only convert cinnamoyl-CoA to vanillin, but its primary role is to catalyse the conversion of hydroxycinnamoyl-CoA to cinnamoyl-CoA, which is a key step in the biosynthesis of lignin.
Books are made of paper, and paper is made from cellulose and lignin. As lignin degrades, it's converted into vanillin, accounting for the hints of vanilla smell that fills up our nose when we inhale deeply an old book’s pages.
Deborah Harrus
About the artwork
Alya Coskun (Year 12) from the Leys School (Cambridge, UK), liked the idea of creating something based on flowers, and found inspiration in vanillin synthase to produce this amazing artwork using etching.
View the artwork in the virtual 2022 PDB Art exhibition.
Structures mentioned in this article
Structure of Hydroxycinnamoyl-CoA Hydratase-Lyase with acetyl-Co A and vanillin, PDB 2VSU
Crystal structure of eugenol oxidase in complex with vanillin, PDB 5FXP
Sources
Hydroxycinnamoyl-CoA hydratase-lyase (Uniprot ID O69762) on PDBe-KB
Maximizing the Efficiency of Vanillin Production by Biocatalyst Enhancement and Process Optimization
Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme


