
A spring herald
Which flowers herald the arrival of spring better than snowdrops? Forming carpets of white, snowdrops mean 'hope' in the language of flowers, as they bloom in early springtime, just before the equinox. Being hardy herbaceous plants, they perennate by underground bulbs, and are among the earliest spring bulbs to bloom, although a few forms can also flower in late autumn.
Snowdrops are native to Europe and the Middle East, and have been known since the earliest times under various names. In 1753, they were named “Galanthus” from the Greek words “gala” (milk) and “anthos” (flower). Galanthus nivalis is the best-known and most widespread representative species of Galanthus, and “nivalis” literally means “of the snow”, referring both to the white drooping bell-shaped flower as well as the fact it can emerge through the snow.
Plant-insect interactions
Plant-insect interactions are complex, and so is the biochemistry between plant proteins and insect proteins. Lectins are proteins that can bind to specific carbohydrates, either simple monosaccharides or more complex glycans, and are at the core of the biochemical relationship between plants and insects. Lectins are often found at high concentrations in certain plant tissues such as seeds, bark, and bulbs, and can serve as defence molecules against insect herbivores or pathogens.
Many plant lectins have strong insecticidal properties, exhibiting toxicity to insect pests by binding with the sugar moieties present in the insect gut, causing agglutination.
Galanthus nivalis agglutinin
The snowdrop’s lectin, Galanthus nivalis agglutinin (GNA) is highly toxic to insects, and has therefore been a focus of agricultural research. In 1998, genetically modified potatoes were created by inserting the GNA gene to give the potatoes resistance to the aphid Myzus persicae. It has also been reported that GNA confers resistance to crewing and sap-sucking insects in rice, tobacco, cotton, rape, and wolfberry without toxicity to higher animals.
GNA is a small 14-kDa mannose-specific lectin from the snowdrop plant. It is a member of the super family of mannose-specific lectins, which can exist as dimers or tetramers. GNA forms 56-kDa homo-tetramers, and is stable at high temperatures (70°C) and over a wide range of pH.
Sugars affect the crystal packing
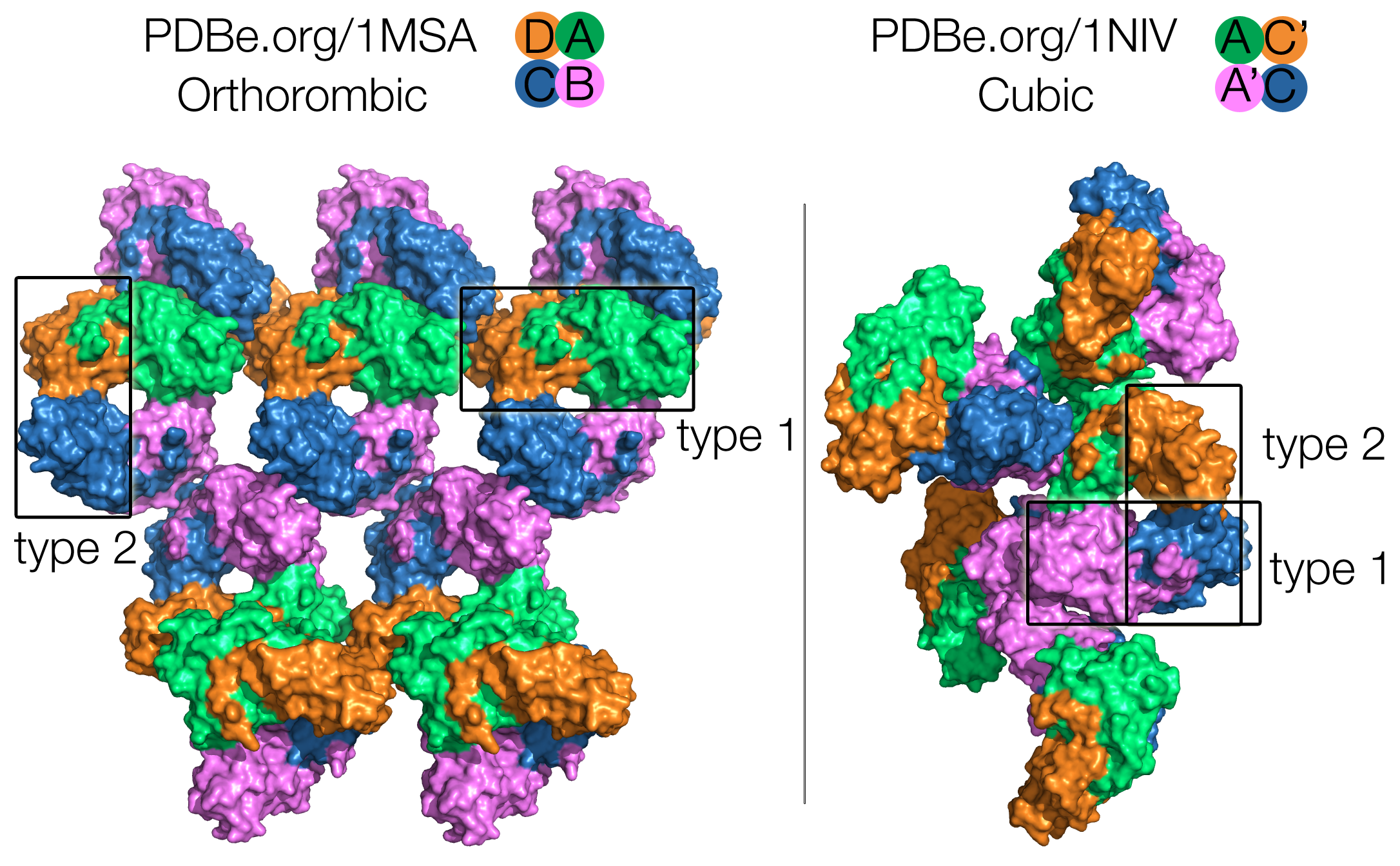
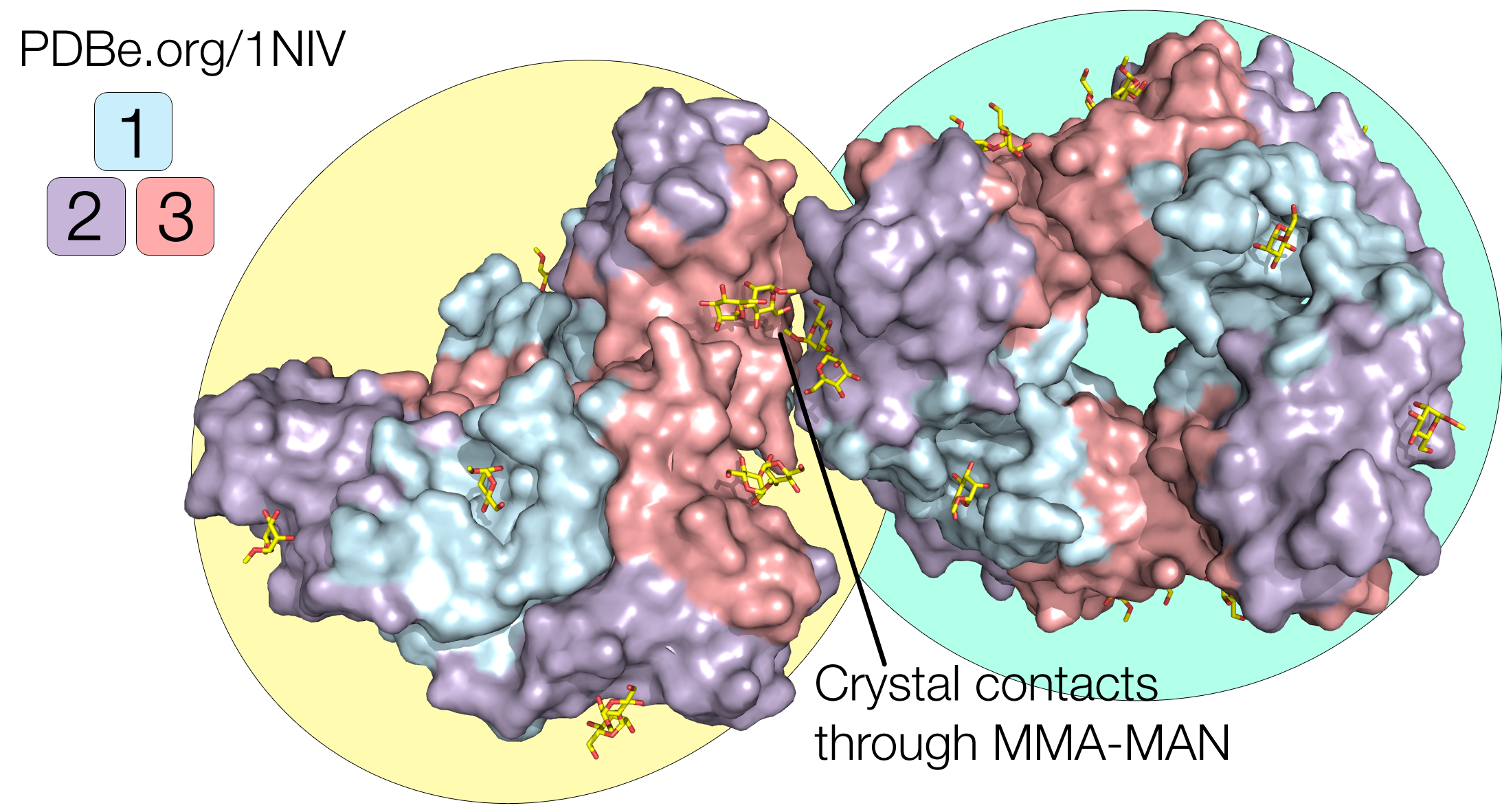
Crystal structures of GNA have been solved in complex with methyl-α-D-mannose (PDB 1MSA, containing MMA), and with mannose-α1,3-D mannose-a-OMe (PDB 1NIV, containing the branched carbohydrate MMA-MAN). Both structures show the GNA in its tetrameric assembly as expected, but an interesting difference between these two structures is that they have two different space groups: P212121 for the orthorhombic crystal structure with MMA, and I213 for the cubic crystal structure with MMA-MAN. This means that the packing interactions are different, with different lattice contacts.
In the orthorhombic crystal structure (1MSA), all four subunits (chains A, B, C, D) of the tetramer are involved in packing interactions. In the cubic crystal structure (1NIV), the bound MMA-MAN mediate crystal packing interactions, and only subunit A (and it’s symmetry-related unit A’) is involved in lattice contacts, while subunit C (and it’s symmetry-related unit C’) is exposed to solvent and is quite mobile (Fig. 1).
Nevertheless the tetramers are identical in both structures, formed by two types of dimers between subunits: type 1 has a wide interface area formed by tight contacts between chains A-D (A-C’) / chains B-C (A’-C); type 2 has a much smaller interface and is formed by contacts between chains A-B (A-A’) / C-D (C-C’). Those two types of inter-subunit contacts exhibit a 222 symmetry (Fig. 1).
A unique and fascinating fold
GNA monomers are composed entirely of β strands, a structural fold that is unique to Amaryllidaceae and unrelated to other lectin families from Gramineae and Leguminoseae for example. Each monomer contains three 4-stranded antiparallel β-sheets related by a quasi 3-fold symmetry. Together, these three β-sheets (termed I, II and III) form a 12-stranded β-barrel with a highly hydrophobic core (Fig. 3). Sheet I is different from sheets II and II, in the fact that it “borrows” its outer strand from the C-terminal end of the neighbouring dimer subunit (Fig. 2).
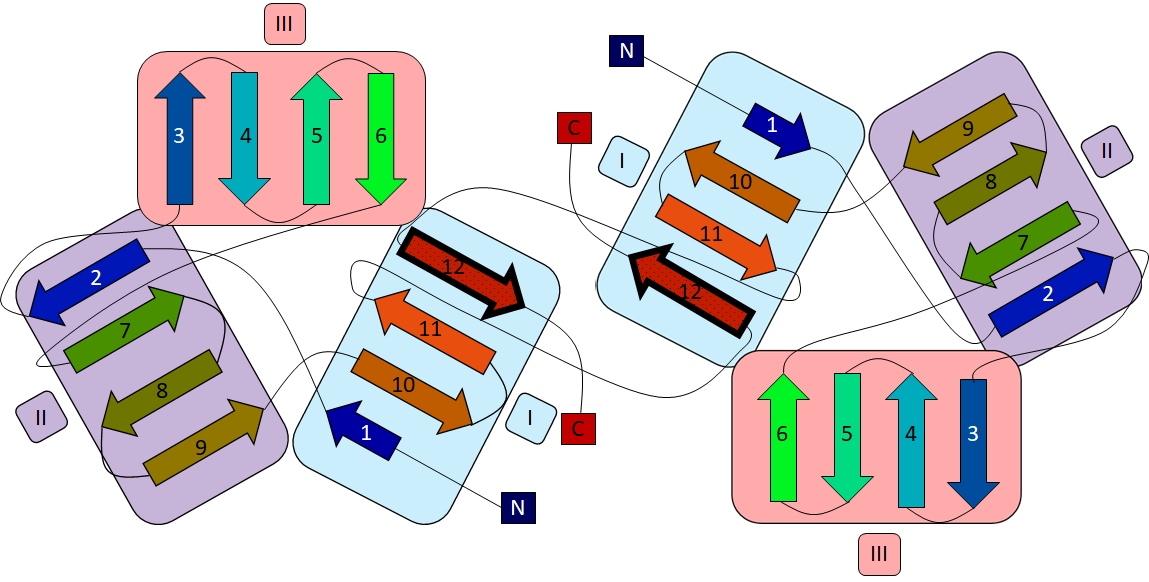
Each of the three β-sheets contain a MMA binding site, termed site 1, 2 and 3 according to their β-sheet location (Fig. 3). Each monomer contains three sites, so the GNA tetramer has a capacity for binding 12 mannose residues.
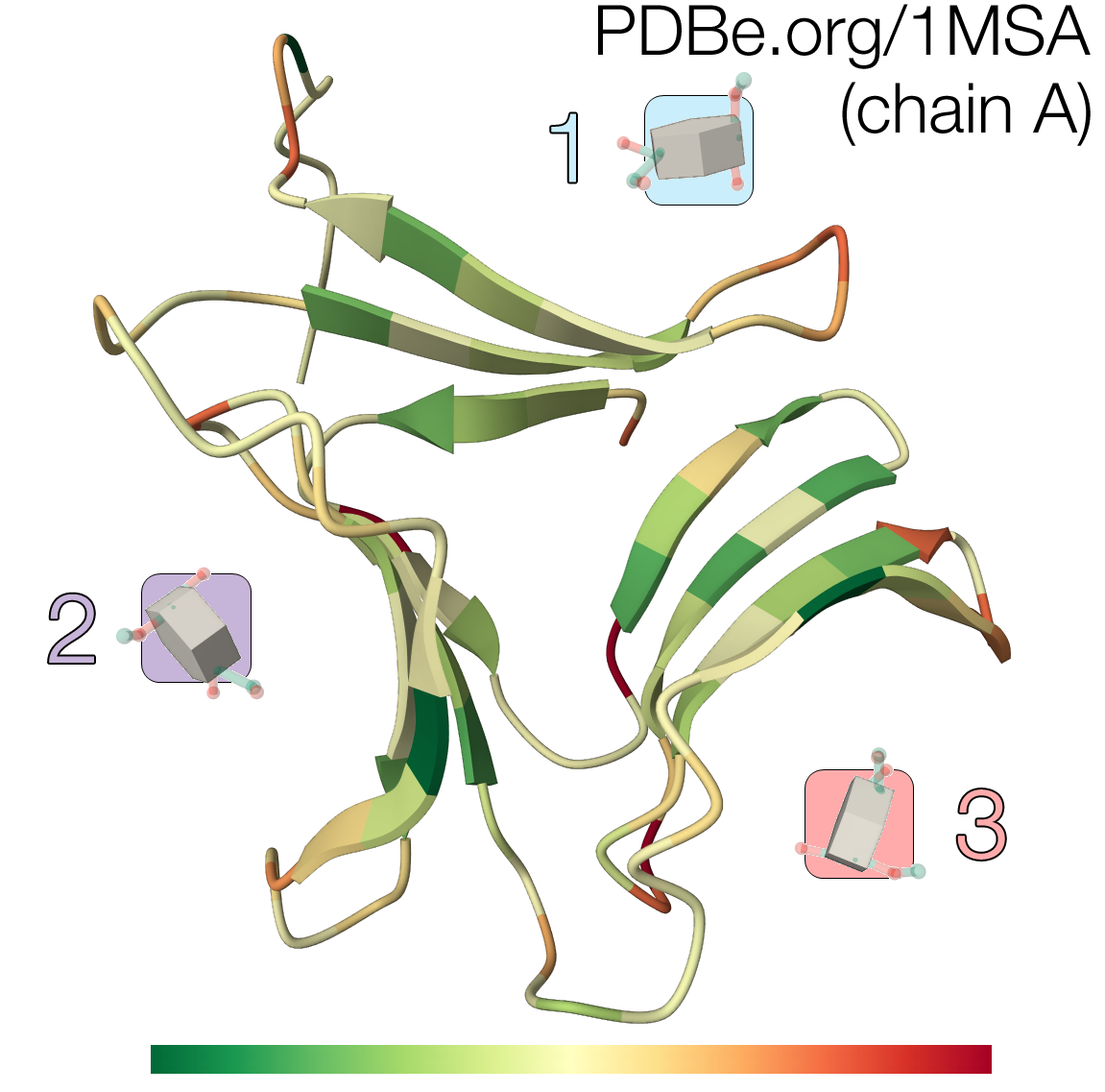
In all three sites, four strictly conserved residues (Asp, Asn, Gln and Tyr) interact with the mannose through five to seven hydrogen bonds, conferring stereospecificity to the binding (Fig. 4). Although all D-mannose recognition and binding sites are completely conserved, MMA only binds with high efficiency at one site (site 1, located in sheet I). This high-affinity binding site coincides with the C-terminal region that stabilizes the inter-subunit interactions by sharing a β-strand to complete the sheet.
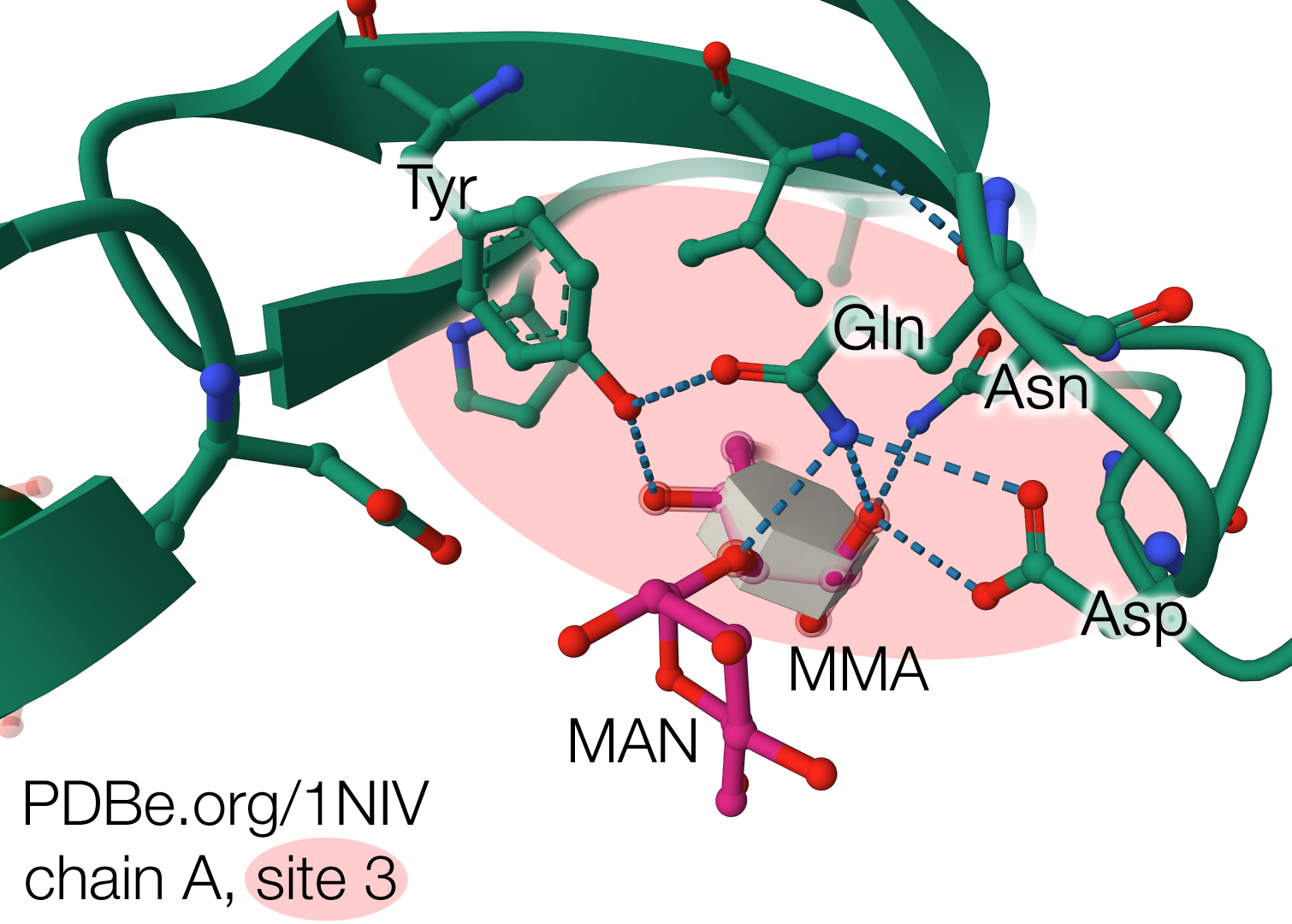
In the structure with MMA-MAN (PDB 1NIV), the di-saccharide bound at site 2 from chain A from one tetramer is in contact with another MMA-MAN bound at site 3 from chain A in the symmetry-related tetramer (Fig. 5). The fact that bound carbohydrates take part in the lattice contacts show that they helped the crystallization process, which commonly happens during crystallization of lectin-saccharide complexes.
The GNA structures in complex with D-mannose (1MSA and 1NIV) are fascinating in many ways: from their unique fold composed exclusively of β strands, the two types of dimer composing the biologically-active tetramer, and the complete conservation of the residues forming the D-mannose recognition domain, at the 3 different binding sites in the β-barrel.
Did you know?
The snowdrop bulbs and flowers also contain an active substance called galantamine, an alkaloid that is an acetylcholinesterase inhibitor. It is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments.
Deborah Harrus
About the artwork
Beatrice Gibbons (Year 12) from the Leys School (Cambridge, UK), chose the snowdrop’s mannose-specific agglutinin because it related to her project on germination and nature. She liked the shape and formation of the protein in the 3D structure, and found that in certain angles it slightly represented the shape of a flower petals, and therefore used it in the overall design of the project. She was also very interested in the Greek origin of the name “Galanthus nivalis”.
For creating her final piece, she photographed a snowdrop to get inspiration for the shape of the overall design. Then she decided to etch the 3D structure of the GNA protein and printed it in different colours onto the calico. She wanted to incorporate the appearance of the snowdrop with the science behind it, so she placed the etching design in the shape of three petals in a snowdrop form. She added embroidery to the calico and the design to outline the petals and add texture and colour to the protein design.
She chose nature colours such as blue and green etching ink and threads to keep the piece connected to nature and plants. She used French knots and other embroidery techniques to add to the 3D aspect of the piece. Additionally, she placed pillow stuffing behind the calico to make the flower pop out the board.
View the artwork in the virtual 2022 PDB Art exhibition.
Structures, proteins and ligands mentioned in this article
Structure of GNA in complex with methyl-α-D-mannose (MMA) (PDB 1MSA)
Structure of GNA in complex with mannose-α1,3-D mannose-a-OMe (MMA-MAN) (PDB 1NIV)
Galanthus nivalis mannose-specific lectin (PDBe-KB P30617)
Sources
Biotechnology for Sustainable Agriculture Chapter 4 - Plant Biotechnology and Crop Improvement
Galanthus nivalis, an overview
Genetically Modified Plants Chapter 3 - Risk Assessment and Management – Human and Animal Health
Plant-insect interactions: what can we learn from plant lectins?


