Isopentenyl phosphate kinase (IPK)
Isopentenyl phosphate kinase belongs to the amino acid kinase (AAK) superfamily and catalyses the phosphorylation reactions that yield isopentenyl diphosphate. It is both a magnesium ion- and ATP-dependent enzyme. Isopentenyl diphosphate is an attractive alternative to the common non-renewable fuels and hence research on this enzyme provides a promising approach for the production of renewable and environmentally friendly biofuels.
Reference Protein and Structure
- Sequence
-
Q9HLX1
 (2.7.4.26)
(2.7.4.26)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Thermoplasma acidophilum DSM 1728 (Archaea)

- PDB
-
3lkk
- Crystal structure of the isopentenyl phosphate kinase substrate complex
(2.001 Å)



- Catalytic CATH Domains
-
3.40.1160.10
 (see all for 3lkk)
(see all for 3lkk)
- Cofactors
- Magnesium(2+) (1)
Enzyme Mechanism
Introduction
The reaction proceeds through a single SN2 step where the phosphate of isopentenyl phosphate attacks the terminal phosphate of ATP.
Catalytic Residues Roles
| UniProt | PDB* (3lkk) | ||
| Lys14 | Lys14(18)A | Part of the catalytic motif: lysine triangle. | electrostatic stabiliser, transition state stabiliser |
| Lys5, Lys14, Lys205 | Lys5(9)A, Lys14(18)A, Lys205(209)A | Form a catalytic motif: lysine triangle. | electrostatic stabiliser, transition state stabiliser |
| His50 | His50(54)A | Forms H-bonds with both the phosphate group of IP and the γ- phosphate of ATP, which provides a favourable conformation for phosphoryl transfer. | electrostatic interaction, electrostatic stabiliser, transition state stabiliser |
| Asp144 | Asp144(148)A | Asp144 forms ionic interactions with Lys5 and Lys205. | electrostatic stabiliser, transition state stabiliser |
| Thr163 | Thr163(167)A | Thr163 stabilises ATP via H-bond. Thr163 points away from the catalytic site, which represents an inactive configuration. Thr163 turns toward ATP to form an H-bond with the β-phosphate and a water molecule, further stabilising ATP so as to facilitate the phosphorylation. | hydrogen bond donor, transition state stabiliser |
| Gly45 (main-N) | Gly45(49)A (main-N) | Forms H-bond with the phosphate group of IP and stabilises the transition states and phosphorylated products. | hydrogen bond donor, transition state stabiliser |
| Gly8 (main-N) | Gly8(12)A (main-N) | Gly8 and Lys14 stabilise the transition state. | hydrogen bond donor, transition state stabiliser |
*PDB label guide - RESx(y)B(C) - RES: Residue Name; x: Residue ID in PDB file;
y: Residue ID in PDB sequence if different from PDB file; B: PDB Chain;
C: Biological Assembly Chain if different from PDB. If label is "Not Found" it means this residue is not found in the reference PDB.
Chemical Components
bimolecular nucleophilic addition, overall reactant used, overall product formedReferences
- McClory J et al. (2017), J Phys Chem B, 121, 11062-11071. Reaction Mechanism of Isopentenyl Phosphate Kinase: A QM/MM Study. DOI:10.1021/acs.jpcb.7b08770. PMID:29155589.
- McClory J et al. (2019), J Phys Chem B, 123, 2844-2852. Phosphorylation Mechanism of N-Acetyl-l-glutamate Kinase, a QM/MM Study. DOI:10.1021/acs.jpcb.9b00547. PMID:30848915.
- Dellas N et al. (2010), ACS Chem Biol, 5, 589-601. Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates. DOI:10.1021/cb1000313. PMID:20392112.
- Mabanglo M et al. (2010),Crystal structure of the isopentenyl phosphate kinase substrate complex. DOI:10.2210/pdb3lkk/pdb.

Step 1. ATP performs a nucleophilic attack on isopentenyl phosphate, forming isopentenyl diphosphate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gly45(49)A (main-N) | transition state stabiliser |
| Lys14(18)A | transition state stabiliser |
| Gly8(12)A (main-N) | transition state stabiliser |
| Lys5(9)A | electrostatic stabiliser |
| Lys14(18)A | electrostatic stabiliser |
| Lys205(209)A | electrostatic stabiliser |
| His50(54)A | electrostatic stabiliser |
| Asp144(148)A | electrostatic stabiliser |
| His50(54)A | electrostatic interaction |
| Thr163(167)A | hydrogen bond donor |
| Lys5(9)A | transition state stabiliser |
| Lys205(209)A | transition state stabiliser |
| His50(54)A | transition state stabiliser |
| Asp144(148)A | transition state stabiliser |
| Thr163(167)A | transition state stabiliser |
| Gly8(12)A (main-N) | hydrogen bond donor |
| Gly45(49)A (main-N) | hydrogen bond donor |

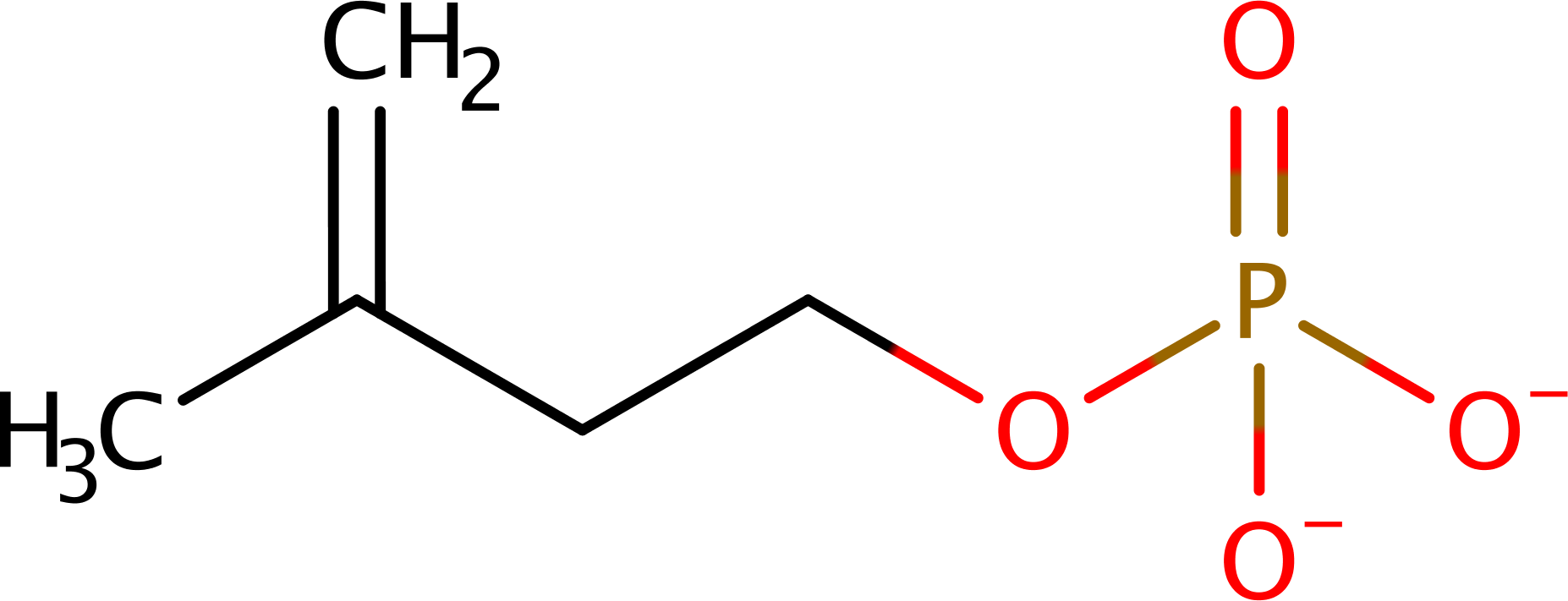


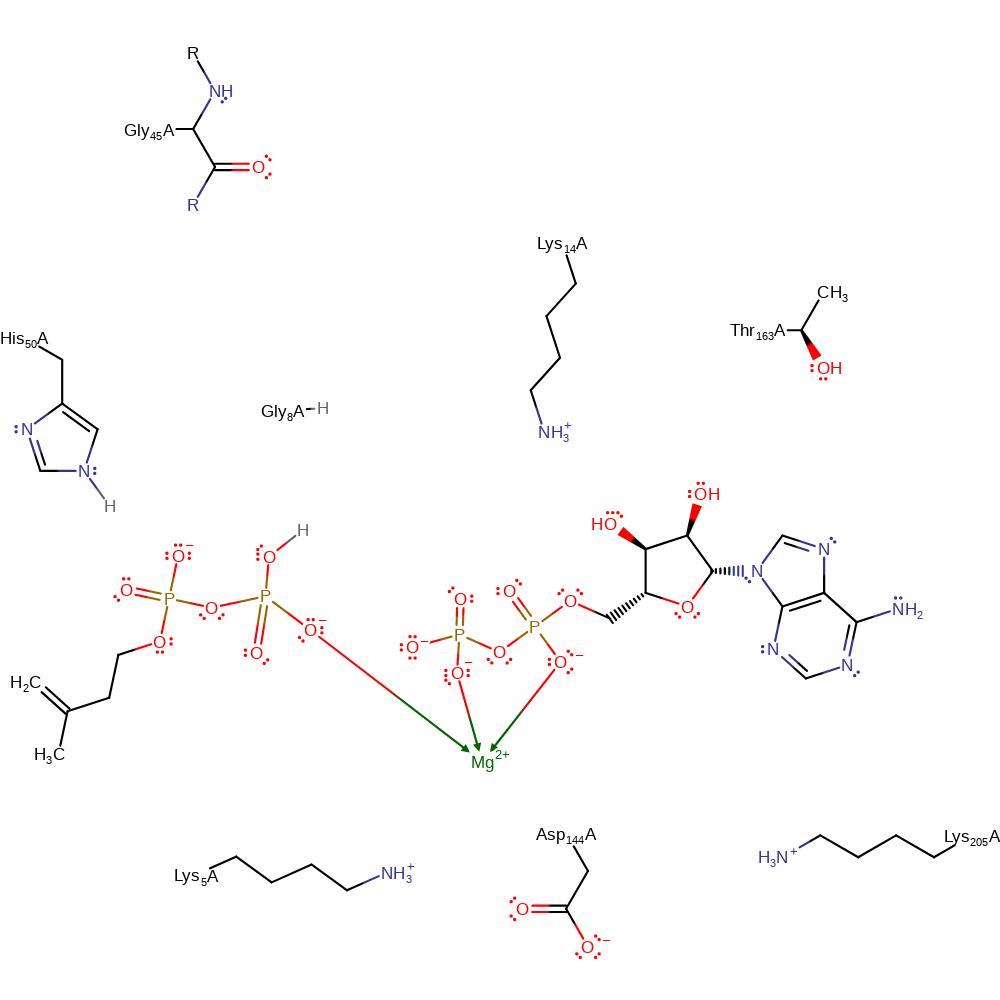 Download:
Download: