Glutathione synthase (prokaryotic)
Glutathione synthetase catalyses the ligation of glycine to gamma-L-glutamyl-L-cysteine (gamma-Glu-Cys) with concomitant hydrolysis of ATP. This is the second and final step in the synthesis of glutathione. Glutathione has many important functions in the cell, including protection against oxidative damage, detoxification of harmful substances, and as a coenzyme for several reactions. Glutathione synthetase is present in species ranging from bacteria to mammals. This entry represents the glutathione synthetases found in Gram-negative bacteria. This gene does not appear to be present in genomes of Gram-positive bacteria.
Reference Protein and Structure
- Sequence
-
P04425
 (6.3.2.3)
(6.3.2.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli K-12 (Bacteria)

- PDB
-
1gsa
- STRUCTURE OF GLUTATHIONE SYNTHETASE COMPLEXED WITH ADP AND GLUTATHIONE
(2.0 Å)



- Catalytic CATH Domains
-
3.30.1490.20
 3.30.470.20
3.30.470.20  (see all for 1gsa)
(see all for 1gsa)
- Cofactors
- Magnesium(2+) (2) Metal MACiE
Enzyme Reaction (EC:6.3.2.3)
Enzyme Mechanism
Introduction
The reaction proceeds via an enzyme-bound acylphosphate intermediate. In the first step, a phosphate group is transferred from ATP to the carboxyl group of the Cys in gamma-Glu-Cys. Arg 225, Lys 160, and two Mg(II) ions may stabilise negative charge in the transition state of phosphoryl transfer. In the second step, the carbonyl of the acyl phosphate is attacked by the amino group of glycine to form a tetrahedral intermediate that is proposed to be stabilised by the guanidinium group of Arg 210. Collapse of the tetrahedral intermediate with loss of phosphate completes the reaction.
Catalytic Residues Roles
| UniProt | PDB* (1gsa) | ||
| Arg210 | Arg210A | Provides a positive charge to stabilise the tetrahedral intermediate resulting from attack by glycine on the carbonyl of the acylphosphate intermediate. | hydrogen bond donor, electrostatic stabiliser |
| Arg225, Lys160 | Arg225A, Lys160A | Proposed to stabilise negative charge in the transition state for phosphoryl transfer. | hydrogen bond donor, electrostatic stabiliser |
| Lys125, Arg233 | Lys125A, Arg233A | Involved in binding and stabilising the negatively charged substrates, intermediates and transition states. | electrostatic stabiliser |
| Asp273 | Asp273A | Forms part of the first magnesium binding site. | metal ligand |
| Glu281 | Glu281A | Acts as a bridging ligand between the two magnesium binding sites. | metal ligand |
| Asn283 | Asn283A | Forms part of the second magnesium binding site. | metal ligand |
Chemical Components
bimolecular nucleophilic substitution, bimolecular nucleophilic addition, proton transfer, unimolecular elimination by the conjugate baseReferences
- Hara T et al. (1996), Biochemistry, 35, 11967-11974. A Pseudo-Michaelis Quaternary Complex in the Reverse Reaction of a Ligase: Structure ofEscherichia coliB Glutathione Synthetase Complexed with ADP, Glutathione, and Sulfate at 2.0 Å Resolution†,‡. DOI:10.1021/bi9605245. PMID:8810901.
- Tanaka T et al. (1997), Arch Biochem Biophys, 339, 151-156. Nicked Multifunctional Loop of Glutathione Synthetase Still Protects the Catalytic Intermediate. DOI:10.1006/abbi.1996.9821. PMID:9056244.
- Matsuda K et al. (1996), Protein Eng, 9, 1083-1092. Crystal structure of glutathione synthetase at optimal pH: domain architecture and structural similarity with other proteins. PMID:9010922.
- Tanaka T et al. (1993), Biochemistry, 32, 12398-12404. Flexibility impaired by mutations revealed the multifunctional roles of the loop in glutathione synthetase. PMID:8241129.
- Yamaguchi H et al. (1993), J Mol Biol, 229, 1083-1100. Three-dimensional Structure of the Glutathione Synthetase from Escherichia coli B at 2·0 Å Resolution. DOI:10.1006/jmbi.1993.1106. PMID:8445637.
- Tanaka T et al. (1992), Biochemistry, 31, 2259-2265. Mutational and proteolytic studies on a flexible loop in glutathione synthetase from Escherichia coli B: the loop and arginine 233 are critical for the catalytic reaction. PMID:1540581.
- Kato H et al. (1988), J Biol Chem, 263, 11646-11651. Role of cysteine residues in glutathione synthetase from Escherichia coli B. Chemical modification and oligonucleotide site-directed mutagenesis. PMID:3042775.

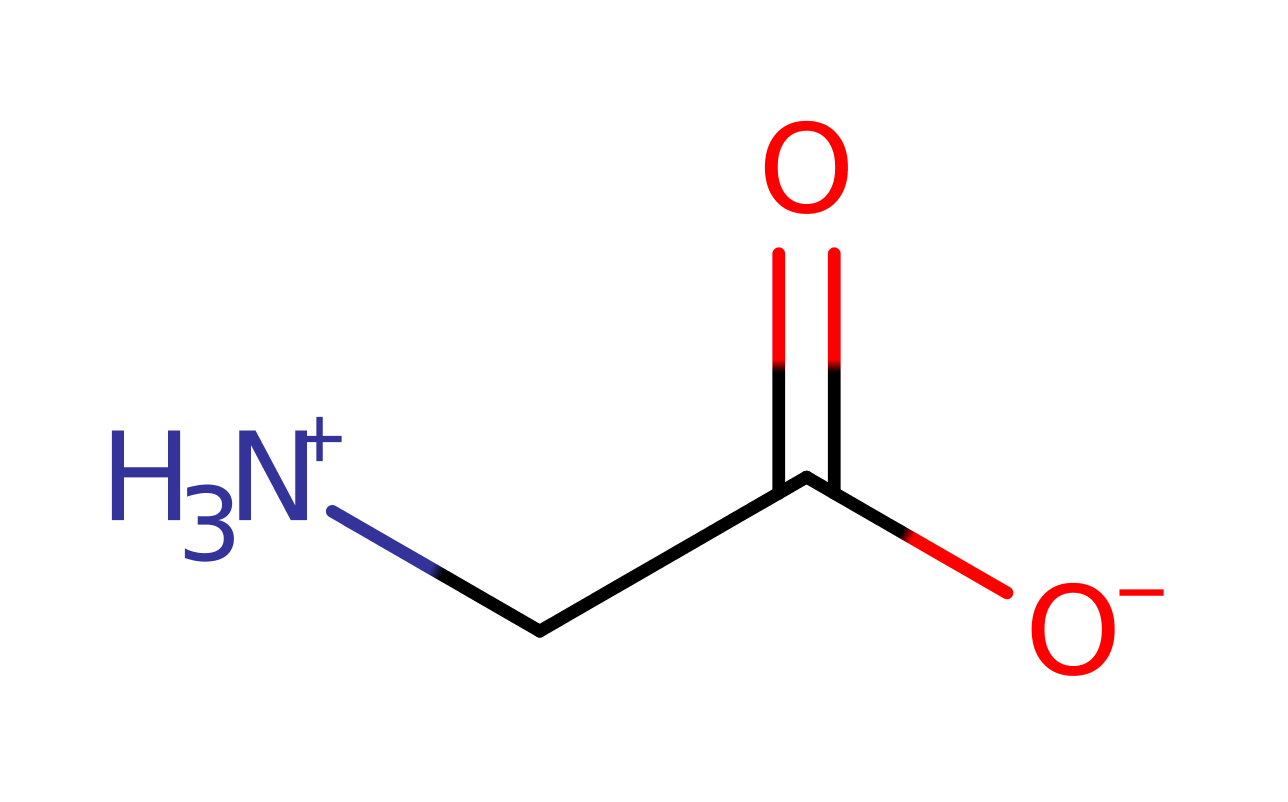
Step 1. The carboxylate group of gamma-L-glutamyl-L-cysteine acts as a nucleophile and attacks the alpha-phoposphate of ATP in a substitution reaction, liberating ADP. The two Mg(II) ions, Lys160, Arg210 and Arg225 all stabilise the intermediates formed.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys160A | hydrogen bond donor, electrostatic stabiliser |
| Arg210A | hydrogen bond donor, electrostatic stabiliser |
| Arg225A | hydrogen bond donor, electrostatic stabiliser |
| Glu281A | metal ligand |
| Asp273A | metal ligand |
| Asn283A | metal ligand |
| Arg233A | electrostatic stabiliser |
| Lys125A | electrostatic stabiliser |
Chemical Components
ingold: bimolecular nucleophilic substitution
Step 2. The amine group of L-glycine acts as a nucleophile and attacks the carbonyl group of the phosphorylated substrate in an addition reaction. The two Mg(II) ions, Lys160, Arg210 and Arg225 all stabilise the intermediates formed.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys160A | hydrogen bond donor, electrostatic stabiliser |
| Arg210A | hydrogen bond donor, electrostatic stabiliser |
| Arg225A | hydrogen bond donor, electrostatic stabiliser |
| Lys125A | electrostatic stabiliser |
| Arg233A | electrostatic stabiliser |
| Glu281A | metal ligand |
| Asp273A | metal ligand |
| Asn283A | metal ligand |
Chemical Components
ingold: bimolecular nucleophilic addition
Step 3. The oxyanion formed re-forms the carbonyl group, cleaving the P-O bond in a conjugate base elimination reaction. The leaving phosphate group deprotonates the newly formed secondary amine. The two Mg(II) ions, Lys160, Arg210 and Arg225 all stabilise the intermediates formed.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys160A | hydrogen bond donor, electrostatic stabiliser |
| Arg210A | hydrogen bond donor, electrostatic stabiliser |
| Arg225A | hydrogen bond donor, electrostatic stabiliser |
| Lys125A | electrostatic stabiliser |
| Arg233A | electrostatic stabiliser |
| Glu281A | metal ligand |
| Asp273A | metal ligand |
| Asn283A | metal ligand |






 Download:
Download: