Carbonate dehydratase (alpha class)
Carbonic acid anhydrase is able to catalyse the conversion of carbon dioxide to carbonic acid and vice versa. This is important in metabolism because it enables the creation of carbonic acid, a very important buffer in the blood which has a vital role in human physiology.
Reference Protein and Structure
- Sequence
-
P00918
 (4.2.1.1)
(4.2.1.1)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Homo sapiens (Human)

- PDB
-
1ca2
- REFINED STRUCTURE OF HUMAN CARBONIC ANHYDRASE II AT 2.0 ANGSTROMS RESOLUTION
(2.0 Å)



- Catalytic CATH Domains
-
3.10.200.10
 (see all for 1ca2)
(see all for 1ca2)
- Cofactors
- Zinc(2+) (1) Metal MACiE
Enzyme Reaction (EC:4.2.1.1)
Enzyme Mechanism
Introduction
The reaction proceeds in two steps. First, an OH- bound to the Zinc ion attacks the carbon dioxide substrate forming bicarbonate and leaving a water at the Zinc ion. Polarisation of the C=O bond of carbon dioxide is achieved by Thr 199. Following this step, the OH- is regenerated by deprotonation of a water molecule by His 64, with Zinc acting to increase the polarity of the OH bond by withdrawing electrons from the oxygen atom.
Catalytic Residues Roles
| UniProt | PDB* (1ca2) | ||
| Thr198 | Thr199(197)A | The interaction between the Thr199 and hydroxide serve to enhance the nucleophilicity of the hydroxide and help orient the substrate (CO2) in the active site. | hydrogen bond acceptor, hydrogen bond donor, activator, electrostatic stabiliser, increase nucleophilicity |
| Glu106 | Glu106(105)A | Hydrogen bonds to the hydroxyl of Thr199, activating it | activator, hydrogen bond acceptor, electrostatic stabiliser |
| His64 | His64(63)A | Is able to deprotonate a water molecule allowing the formation of the catalytic nucleophile OH-. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| His94, His96, His119 | His94(93)A, His96(95)A, His119(118)A | Forms part of the catalytic zinc binding site. | metal ligand |
Chemical Components
bimolecular nucleophilic addition, overall reactant used, overall product formed, coordination to a metal ion, proton transfer, native state of enzyme regeneratedReferences
- Fisher Z et al. (2005), Biochemistry, 44, 1097-1105. Structural and Kinetic Characterization of Active-Site Histidine as a Proton Shuttle in Catalysis by Human Carbonic Anhydrase II†,‡. DOI:10.1021/bi0480279. PMID:15667203.
- Supuran CT et al. (2003), Med Res Rev, 23, 146-189. Carbonic anhydrase inhibitors. DOI:10.1002/med.10025. PMID:12500287.
- Huang S et al. (1998), J Mol Biol, 283, 301-310. Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. DOI:10.1006/jmbi.1998.2077. PMID:9761692.
- Lindskog S (1997), Pharmacol Ther, 74, 1-20. Structure and mechanism of carbonic anhydrase. DOI:10.1016/s0163-7258(96)00198-2. PMID:9336012.
- Tu CK et al. (1989), Biochemistry, 28, 7913-7918. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. DOI:10.1021/bi00445a054. PMID:2514797.
- Eriksson AE et al. (1988), Proteins, 4, 274-282. Refined structure of human carbonic anhydrase II at 2.0 Å resolution. DOI:10.1002/prot.340040406. PMID:3151019.
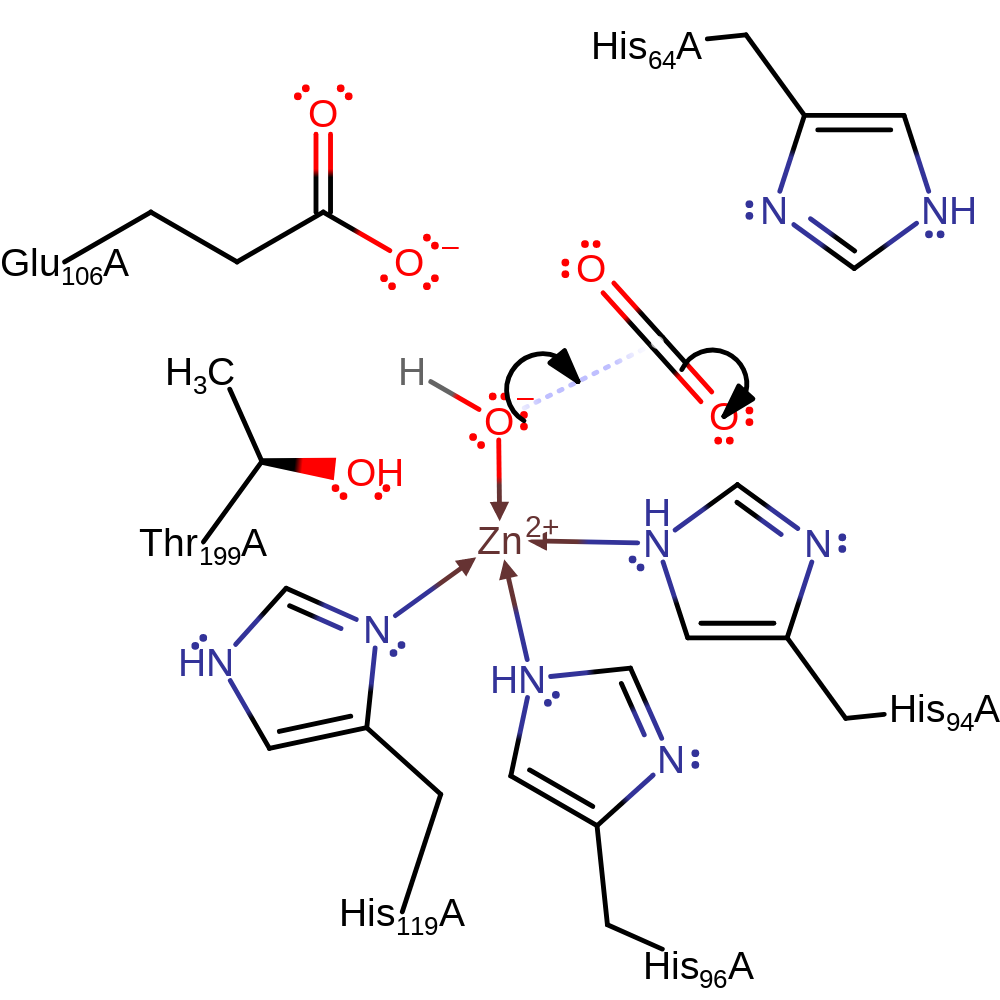
Step 1. The interactions between the zinc bound hydroxide, Thr199 and Gu106 serve to enhance the nucleophilicity of the hydroxide and help to orient the substrate (carbon dioxide) in the active site [PMID:12500287]. Zinc activated water attacks the carbon of carbon dioxide in a nucleophilic addition, which results in the bicarbonate moiety being bound to the zinc in a bidentate manner.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His119(118)A | metal ligand |
| His94(93)A | metal ligand |
| His96(95)A | metal ligand |
| Glu106(105)A | hydrogen bond acceptor, electrostatic stabiliser, activator |
| Thr199(197)A | hydrogen bond acceptor, hydrogen bond donor, electrostatic stabiliser, activator, increase nucleophilicity |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, overall product formed, coordination to a metal ion
Step 2. The bicarbonate ion formed at the end of the first step is displaced by a water molecules before this step occurs [PMID:12500287]. His64 deprotonates the zinc bound water.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu106(105)A | hydrogen bond acceptor, activator, electrostatic stabiliser |
| Thr199(197)A | hydrogen bond acceptor, hydrogen bond donor, activator, electrostatic stabiliser |
| His64(63)A | hydrogen bond acceptor |
| His119(118)A | metal ligand |
| His94(93)A | metal ligand |
| His96(95)A | metal ligand |
| His64(63)A | proton acceptor |
Chemical Components
proton transfer
Step 3. Evidence suggests that upon receiving the proton from the water, the His64 flips from the "in" configuration to the "out" configuration to deliver its proton to the solution. It is possible there is a longer proton relay chain present [PMID:15667203].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu106(105)A | hydrogen bond acceptor, electrostatic stabiliser |
| Thr199(197)A | hydrogen bond acceptor, hydrogen bond donor, electrostatic stabiliser |
| His64(63)A | hydrogen bond donor |
| His119(118)A | metal ligand |
| His94(93)A | metal ligand |
| His96(95)A | metal ligand |
| His64(63)A | proton donor |




 Download:
Download: