Squalene-hopene cyclase
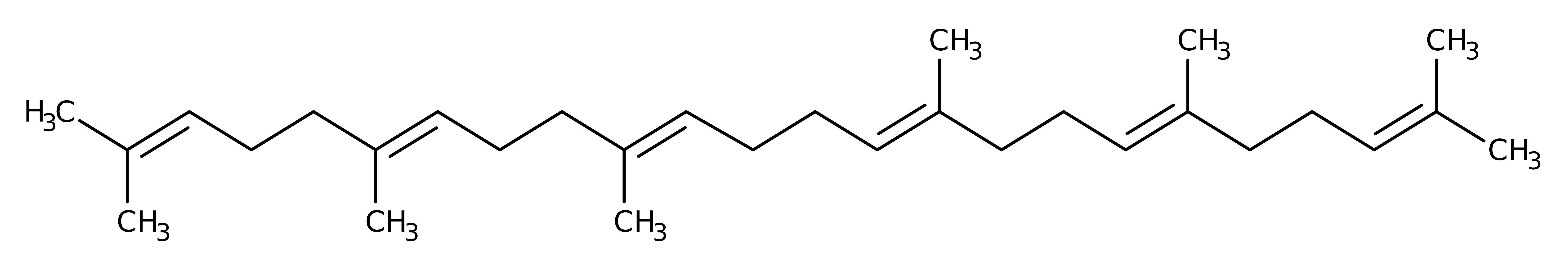
Squalene-hopene cyclase (SHC) from Alicyclobacillus acidocaldarius catalyses the cyclisation of linear triterpene squalene. There are two possible products; hop-22(29)-ene or hopan-22-ol (aka diplopterol) which are formed in a roughly 90:10 ratio. SHC is a membrane protein with characteristics similar to those of prostaglandin-H2 synthase, the only other reported protein of this type. Enzymes like SHC are the targets for the development of anticholesteremic and antifungal drugs.
Reference Protein and Structure
- Sequence
-
P33247
 (4.2.1.129, 5.4.99.17)
(4.2.1.129, 5.4.99.17)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Alicyclobacillus acidocaldarius subsp. acidocaldarius DSM 446 (Bacteria)

- PDB
-
1h3b
- SQUALENE-HOPENE CYCLASE
(2.8 Å)



- Catalytic CATH Domains
-
1.50.10.20
 (see all for 1h3b)
(see all for 1h3b)
Enzyme Reaction (EC:5.4.99.17)
Enzyme Mechanism
Introduction
The general acid (protonated) Asp 376 (coupled with His 451) protonates the substrate squalene at C3, forming a carbocation, and leaving the Asp:His diad as a stable salt bridge. The acidity of Asp 376 is increased by Tyr 495. The positive charge on the Asp:His couple is stabilised by a negative charge on the Asp 374, Asp 377 couple. There is a cyclization cascade, and the cation makes its way down the molecule. Atoms C4, C10, C8, C13, C17, C18 and C22 are stabilised by the residues Trp 312, Trp 489, Phe 365, Trp 169 and Phe 601 respectively (Phe 601 stabilising 3 positions.) At C29, a water molecule acts as a base by accepting a proton from the substrate to form hopene. This water molecule is polarised by other waters which are in turn polarised by a network of hydrogen bonds between Gln 262, Glu 45, Glu 93 and Arg 127. Diplopterol is formed if the front water adds as hydroxyl to the last carbocation instead of accepting the proton. Asp 376 is re-protonated by Tyr 495 via a water molecule, the proton originating from solvent water. The carbon numbering used above is for hopene.
Catalytic Residues Roles
| UniProt | PDB* (1h3b) | ||
| Asp374 | Asp374A | Works with Asp 377 in stabilising the positive charge of the Asp 376, His 451 couple. | modifies pKa |
| Phe365 | Phe365A | Stabilises the carbocation in the C8 position. | van der waals interaction, electrostatic stabiliser |
| Asp376 | Asp376A | Acts as a general acid (along with His 451) to protonate the C3 atom of the substrate. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| His451 | His451A | Assists Asp 376 in protonating the C3 atom of the substrate. | hydrogen bond donor, electrostatic stabiliser |
| Trp489 | Trp489A | Stabilises the carbocation in the C10 position | van der waals interaction, electrostatic stabiliser, polar/non-polar interaction |
| Tyr495 | Tyr495A | Tyr 495 increases the acidity of Asp 376, making it a better general acid. It also re-protonates Asp 376 via a water molecule. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, proton relay, electrostatic stabiliser |
| Phe601 | Phe601A | Stabilises the carbocation in the C18 position. | van der waals interaction, electrostatic stabiliser |
| Phe605 | Phe605A | Stabilises the carbocation in the C17 and C22 positions. | van der waals interaction, electrostatic stabiliser |
| Tyr609 | Tyr609A | The conserved residue Tyr 609 helps to stabilise the carbocation during the course of the reaction. | van der waals interaction, electrostatic stabiliser, polar/non-polar interaction |
| Trp169 | Trp169A | Stabilises the carbocation in the C13 position. | van der waals interaction, electrostatic stabiliser, polar/non-polar interaction |
| Tyr420 | Tyr420A | Helps stabilise the reactive intermediate formed during the course of the reaction. | van der waals interaction, electrostatic stabiliser, polar/non-polar interaction |
| Glu45, Glu93, Arg127, Gln262 | Glu45A, Glu93A, Arg127A, Gln262A | Helps to polarise the water molecule which acts as a general base at the end of the mechanism. | increase basicity, modifies pKa, hydrogen bond acceptor |
| Trp312 | Trp312A | Stabilises the carbocation in the C4 position. | electrostatic stabiliser |
| Asp377 | Asp377A | Works with Asp 374 in stabilising the positively charged Asp 376, His 451 couple. | modifies pKa |
Chemical Components
proton transfer, intermediate formation, overall reactant used, intramolecular electrophilic addition, cyclisation, overall product formed, intermediate terminated, native state of enzyme regenerated, proton relayReferences
- Wendt KU et al. (1999), J Mol Biol, 286, 175-187. The structure of the membrane protein squalene-hopene cyclase at 2.0 å resolution. DOI:10.1006/jmbi.1998.2470. PMID:9931258.
- Takahashi K et al. (2018), European J Org Chem, 2018, 1477-1490. Squalene-Hopene Cyclase: On the Polycyclization Reactions of Squalene Analogues Bearing Ethyl Groups at Positions C-6, C-10, C-15, and C-19. DOI:10.1002/ejoc.201800010.
- Abe I (2007), Nat Prod Rep, 24, 1311-1331. Enzymatic synthesis of cyclic triterpenes. DOI:10.1039/b616857b. PMID:18033581.
- Wendt KU et al. (1997), Science, 277, 1811-1815. Structure and Function of a Squalene Cyclase. DOI:10.1126/science.277.5333.1811. PMID:9295270.

Step 1. Protonation of the terminal double bond, by Asp376, initiates the reaction cascade [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Trp169A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Phe605A | electrostatic stabiliser |
| Tyr609A | electrostatic stabiliser |
| Asp376A | hydrogen bond donor, hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor, modifies pKa |
| Glu93A | modifies pKa |
| Arg127A | modifies pKa |
| Gln262A | modifies pKa |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
| Asp376A | proton donor |
Chemical Components
proton transfer, intermediate formation, overall reactant used
Step 2. An intramolecular Markovnikov-type cation–olefin addition first produces a 6.6 bicyclic tertiary cation, which is stabilised by the pi-electrons and dipoles of aromatic residues including Phe365, Tyr420, Trp489, and Tyr609 [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction, electrostatic stabiliser |
| Tyr420A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Trp489A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Tyr609A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
| Trp169A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Phe605A | electrostatic stabiliser |
| Glu45A | modifies pKa |
| Glu93A | modifies pKa |
| Arg127A | modifies pKa |
| Gln262A | modifies pKa |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation
Step 3. The initial cyclisation cascade to form the 6.6.6.5 tetracyclic intermediate cation is thought to proceed almost concomitantly and without significant conformational changes. Then, a six-membered C-ring is formed directly in concert with formation of a Markovnikov-type five-membered D-ring, generating a 6.6.6.5-fused tetracyclic intermediate tertiary cation, which is stabilized by Phe601, Phe605, and Trp169 through cation–pi interactions. The proposed relatively long life time of the 6.6.6.5 tetracyclic intermediate cation seems to be consistent with the experimental observation that 6.6.6.5 tetracyclic products have been obtained as minor products of wild-type SHC, and also as products of various site-directed mutants of SHC [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction, electrostatic stabiliser |
| Phe605A | van der waals interaction, electrostatic stabiliser |
| Trp169A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Glu45A | hydrogen bond acceptor |
| Trp312A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Tyr609A | electrostatic stabiliser |
| Glu45A | modifies pKa |
| Glu93A | modifies pKa |
| Arg127A | modifies pKa |
| Gln262A | modifies pKa |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation
Step 4. The cyclisation reaction is subsequently followed by D-ring expansion and Markovnikov E-ring closure, yielding the tertiary hopanyl C-22 cation with the 6.6.6.6.5-fused pentacyclic ring system, which is likely to be stabilized by the conserved Phe605 pi-electrons [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Trp169A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Tyr609A | electrostatic stabiliser |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction, electrostatic stabiliser |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor, modifies pKa |
| Glu93A | modifies pKa |
| Arg127A | modifies pKa |
| Gln262A | modifies pKa |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation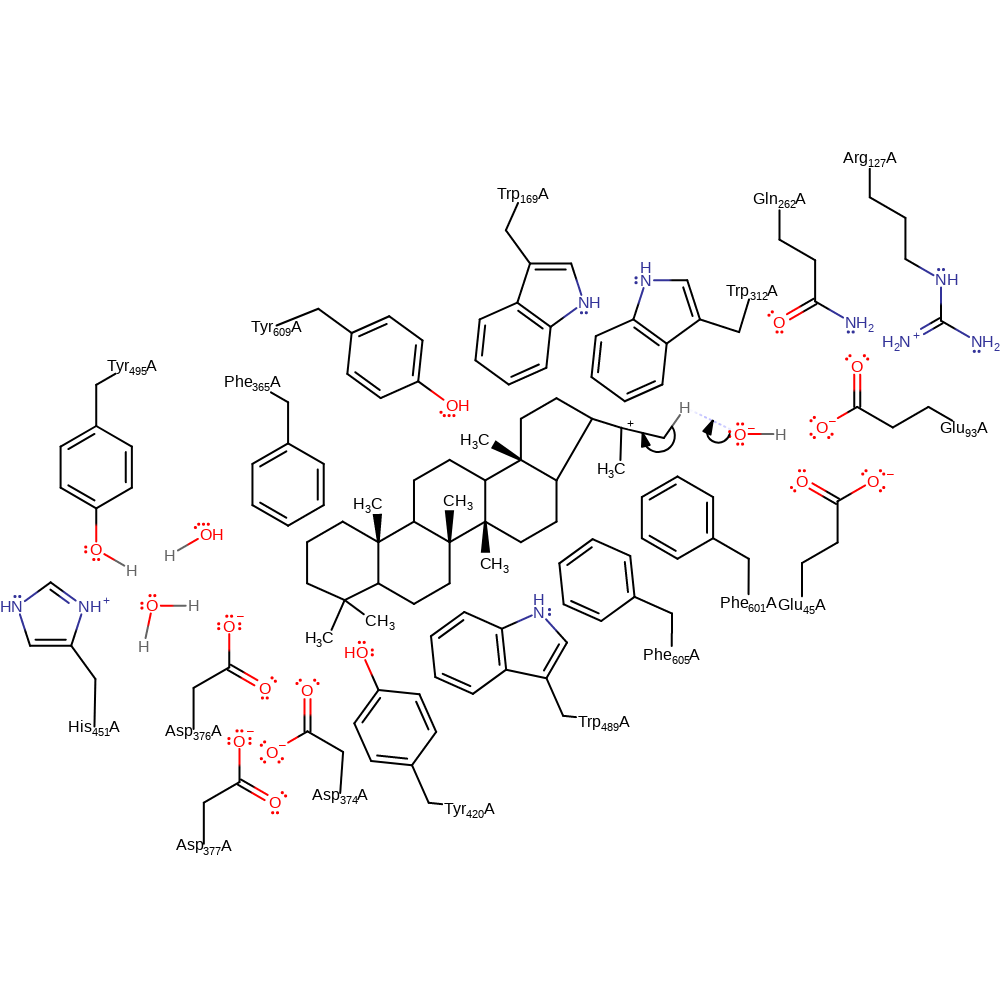
Step 5. Finally, the enzyme reaction is terminated by either regiospecific elimination of the Z-methyl group to the major product hop-22(29)-ene. It has been proposed that the water molecules are polarised by residues in the hydrogen-bonding network around Glu45 at the bottom of the active-site cavity; a polarised water molecule abstracts the proton or attacks the E-ring cation, to terminate the reaction. In fact, the product ratio of hopene and hopanol has been reported to be significantly altered in E45A and E45D mutants of A. acidocaldarius SHC [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor, increase basicity, modifies pKa |
| Glu93A | modifies pKa |
| Arg127A | modifies pKa |
| Gln262A | modifies pKa |
| Trp169A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Phe605A | electrostatic stabiliser |
| Tyr609A | electrostatic stabiliser |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
Chemical Components
proton transfer, overall product formed, intermediate terminated
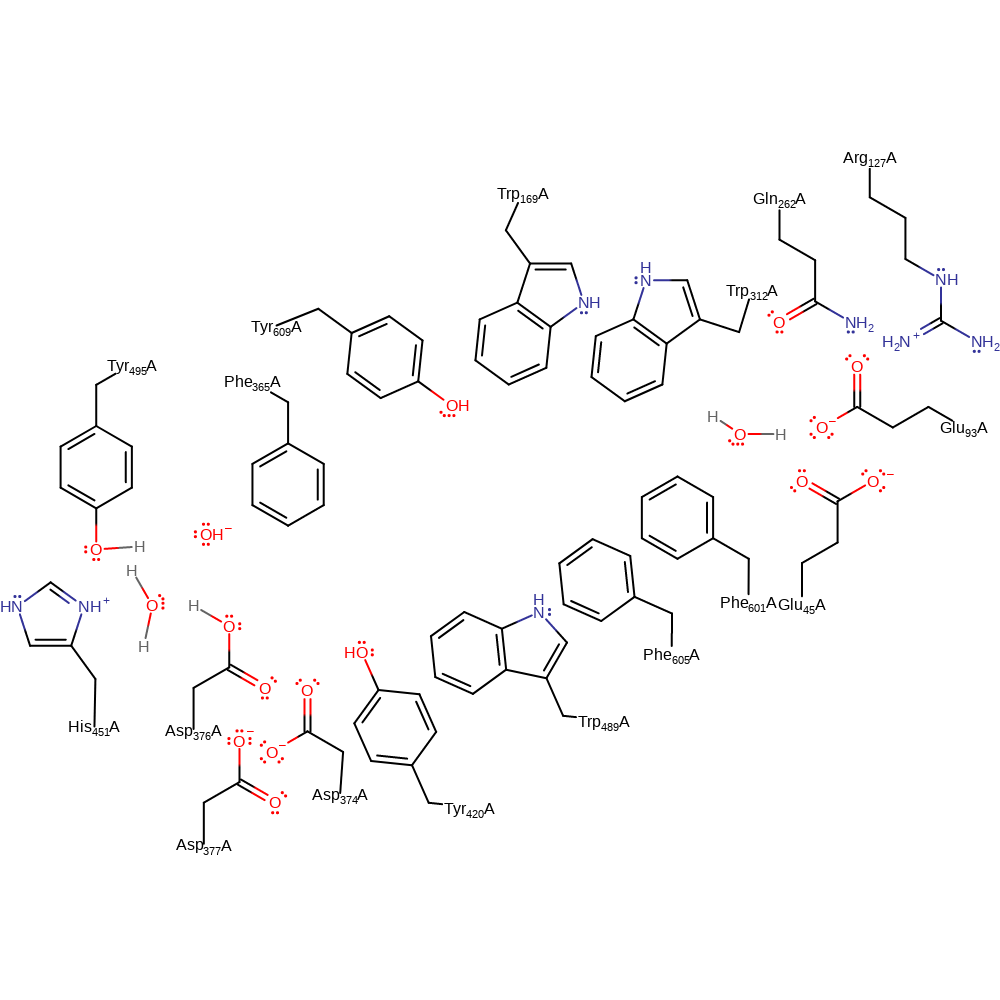
Step 6. Asp376 is reprotonated after turnover via a proton relay chain involving Asp376, Water, Tyr495 and disordered water in the upper cavity of the active site [PMID:9931258].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, hydrogen bond acceptor, proton relay |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
| Asp374A | modifies pKa |
| Asp377A | modifies pKa |
| Tyr495A | proton acceptor, proton donor |
| Asp376A | proton acceptor |
Chemical Components
proton transfer, native state of enzyme regenerated, proton relayIntroduction
This alternative mechanism is the same as the first one but leads to the formation of the minor product when a water attacks the carbocation rather than abstracting a proton.
Catalytic Residues Roles
| UniProt | PDB* (1h3b) |
Chemical Components
proton transfer, intermediate formation, overall reactant used, intramolecular electrophilic addition, cyclisation, overall product formed, intermediate terminated, bimolecular nucleophilic addition, native state of enzyme regenerated, proton relayReferences

Step 1. Protonation of the terminal double bond, by Asp376, initiates the reaction cascade [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr609A | electrostatic stabiliser |
| Phe605A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Trp169A | electrostatic stabiliser |
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Gln262A | modifies pKa |
| Arg127A | modifies pKa |
| Glu93A | modifies pKa |
| Glu45A | modifies pKa |
| Asp376A | hydrogen bond donor, hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
| Asp376A | proton donor |
Chemical Components
proton transfer, intermediate formation, overall reactant used
Step 2. An intramolecular Markovnikov-type cation–olefin addition first produces a 6.6 bicyclic tertiary cation, which is stabilised by the pi-electrons and dipoles of aromatic residues including Phe365, Tyr420, Trp489, and Tyr609 [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Gln262A | modifies pKa |
| Arg127A | modifies pKa |
| Glu93A | modifies pKa |
| Glu45A | modifies pKa |
| Phe605A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Trp169A | electrostatic stabiliser |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction, electrostatic stabiliser |
| Tyr420A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Trp489A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Tyr609A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation
Step 3. The initial cyclisation cascade to form the 6.6.6.5 tetracyclic intermediate cation is thought to proceed almost concomitantly and without significant conformational changes. Then, a six-membered C-ring is formed directly in concert with formation of a Markovnikov-type five-membered D-ring, generating a 6.6.6.5-fused tetracyclic intermediate tertiary cation, which is stabilized by Phe601, Phe605, and Trp169 through cation–pi interactions. The proposed relatively long life time of the 6.6.6.5 tetracyclic intermediate cation seems to be consistent with the experimental observation that 6.6.6.5 tetracyclic products have been obtained as minor products of wild-type SHC, and also as products of various site-directed mutants of SHC [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Gln262A | modifies pKa |
| Arg127A | modifies pKa |
| Glu93A | modifies pKa |
| Glu45A | modifies pKa |
| Tyr609A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction, electrostatic stabiliser |
| Phe605A | van der waals interaction, electrostatic stabiliser |
| Trp169A | van der waals interaction, polar/non-polar interaction, electrostatic stabiliser |
| Glu45A | hydrogen bond acceptor |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation
Step 4. The cyclisation reaction is subsequently followed by D-ring expansion and Markovnikov E-ring closure, yielding the tertiary hopanyl C-22 cation with the 6.6.6.6.5-fused pentacyclic ring system, which is likely to be stabilized by the conserved Phe605 pi-electrons [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr609A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Trp169A | electrostatic stabiliser |
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Gln262A | modifies pKa |
| Arg127A | modifies pKa |
| Glu93A | modifies pKa |
| Glu45A | modifies pKa |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction, electrostatic stabiliser |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
Chemical Components
ingold: intramolecular electrophilic addition, intermediate formation, cyclisation
Step 5. Finally, the enzyme reaction is terminated by either regiospecific elimination of the Z-methyl group to the major product hop-22(29)-ene. It has been proposed that the water molecules are polarised by residues in the hydrogen-bonding network around Glu45 at the bottom of the active-site cavity; a polarised water molecule abstracts the proton or attacks the E-ring cation, to terminate the reaction (as shown here). In fact, the product ratio of hopene and hopanol has been reported to be significantly altered in E45A and E45D mutants of A. acidocaldarius SHC [PMID:18033581].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Tyr609A | electrostatic stabiliser |
| Phe605A | electrostatic stabiliser |
| Phe601A | electrostatic stabiliser |
| Trp489A | electrostatic stabiliser |
| Tyr420A | electrostatic stabiliser |
| Phe365A | electrostatic stabiliser |
| Trp312A | electrostatic stabiliser |
| Trp169A | electrostatic stabiliser |
| Gln262A | modifies pKa |
| Arg127A | modifies pKa |
| Glu93A | modifies pKa |
| Glu45A | modifies pKa |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, electrostatic stabiliser |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor, increase nucleophilicity, proton acceptor |
Chemical Components
proton transfer, overall product formed, intermediate terminated, ingold: bimolecular nucleophilic addition
Step 6. Asp376 is reprotonated after turnover via a proton relay chain involving Asp376, Water, Tyr495 and disordered water in the upper cavity of the active site [PMID:9931258].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asp377A | modifies pKa |
| Asp374A | modifies pKa |
| Asp376A | hydrogen bond acceptor |
| His451A | hydrogen bond donor, electrostatic stabiliser |
| Tyr495A | hydrogen bond donor, hydrogen bond acceptor, proton relay |
| Phe365A | van der waals interaction |
| Tyr420A | van der waals interaction, polar/non-polar interaction |
| Trp489A | van der waals interaction, polar/non-polar interaction |
| Tyr609A | van der waals interaction, polar/non-polar interaction |
| Phe601A | van der waals interaction |
| Phe605A | van der waals interaction |
| Trp169A | van der waals interaction, polar/non-polar interaction |
| Glu45A | hydrogen bond acceptor |
| Tyr495A | proton donor |
| Asp376A | proton acceptor |
| Tyr495A | proton acceptor |

 Download:
Download:  Download:
Download: