5-epi-aristolochene synthase
5-epi-aristolochene synthase from tobacco belongs to the family of terpene cyclases and is a member of the I Isoprenoid Synthase Type I superfamily. 5-epi-aristolochene synthase from tobacco (Nicotiana tabacum) converts farnesyl diphosphate into 5-epi-Aristolochene and diphosphate in a magnesium dependent manner. 5-epi-aristolochene is a precursor of the antifungal phytoalexin capsidiol. Based on structural studies of the tobacco enzyme it is believed the terpene is formed via two intermediates, germacrene and a eudesmane carbocation.
Reference Protein and Structure
- Sequence
-
Q40577
 (4.2.3.61)
(4.2.3.61)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Nicotiana tabacum (common tobacco)

- PDB
-
5eat
- 5-EPI-ARISTOLOCHENE SYNTHASE FROM NICOTIANA TABACUM WITH SUBSTRATE ANALOG FARNESYL HYDROXYPHOSPHONATE
(2.8 Å)



- Catalytic CATH Domains
-
1.10.600.10
 (see all for 5eat)
(see all for 5eat)
- Cofactors
- Magnesium(2+) (3) Metal MACiE
Enzyme Reaction (EC:4.2.3.61)
Enzyme Mechanism
Introduction
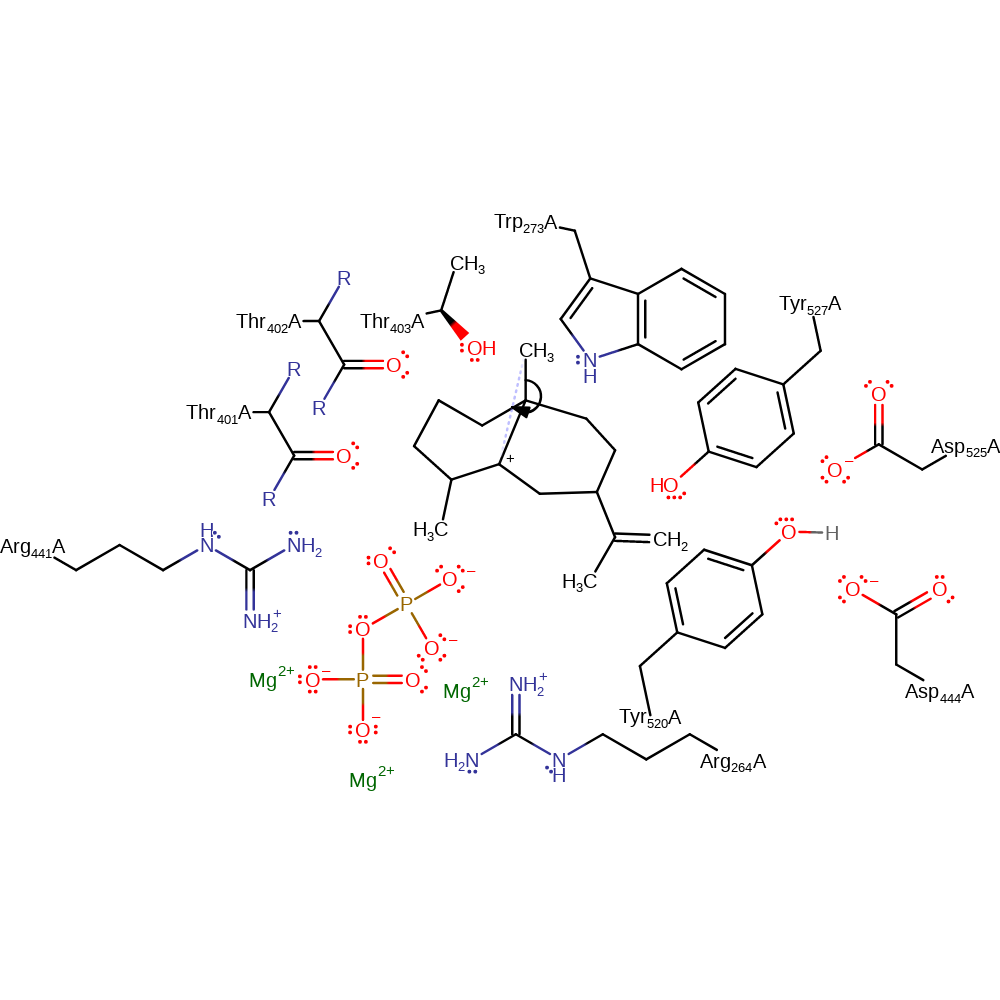
A mechanism is proposed based on crystal structures of the enzyme. The first step of the mechanism involves the departure of diphosphate, generating a carbocation intermediate with the positive charge delocalised over C1, C2 and C3. The additional negative charge of the diphosphate is offset by interactions with the three Mg2+ ions, Arg 264 and Arg 441 while the positive charge of the carbocation is stabilised by Thr401 and Thr402 backbone carbonyls and hydroxyl group of Thr403. The carbocation is then poised for attack on C10. Once C1 has been positioned near the p-orbitals of the C10-C11 bond, electrophilic attack at C10 would create a C1-C10 bond and a tertiary carbocation on C11. The quadrupole of Tyr 527 is nicely positioned to stabilise the positive charge on C11. In turn, the newly formed carbocation at C11 substantially increases the acidity of the C12 and C13 methyl protons. Asp 525 then abstracts a proton from C13 (cis methyl group), leading to the formation of a neutral germacrene intermediate. The next step involves proton addition at C6 by the Asp444-Tyr520-Asp525 triad. The carboxyl group of Asp 444 removes the hydroxyl proton from Tyr520. At the same time, the phenolic oxygen at Tyr520 accepts a proton from Asp525. Tyr520 then donates this newly positioned proton to the double bond of germacrene at C6. In a concerted fashion, Tyr520 reaccepts the proton currently on the Asp444 carboxyl group. Concomitant with the C6 protonation by Tyr 520, germacrene undergoes a second ring closure as the p-orbitals on C2 and C7 line up for the formation of the C2-C7 sigma bond. The resulting bicyclic eudesmane carbocation intermediate with the positive charge on C3 is stabilised by the summed dipoles of the Thr401 and Thr402 main chain carbonyls and Thr403 hydroxyl group.
A carbocation centred on C7 must be formed for subsequent reactions. The C2 hydride first migrates to the planar C3 carbocation along the top of the bicyclic ring system leaving a tertiary carbocation with planar geometry at C2. C7 abuts the aromatic face of Trp 273 and stabilisation by this aromatic quadrupole selectively positions the electrophilic centre at C7. The eudesmane carbocation intermediate with the positive charge centred at C2 exists in a conformation that orients the C7-C14 sigma bond parallel to the empty p-orbital on C2. This conformer facilitates the migration of the C14 methyl group from C7 to C2. The conformation of the resulting carbocation with a positive charge positioned on C7 directs a proton on C8 toward the indole ring of Trp 273. The presence of the carbocation centre on C7 greatly increases the acidity of the proton at C8 which may now be removed by Trp 273, giving rise to a positive arenium ion (Trp H1 at residue 273) and the final product, 5-epi-aristolochene.
Catalytic Residues Roles
| UniProt | PDB* (5eat) | ||
| Thr401 (main-C), Thr402 (main-C) | Thr401A (main-C), Thr402A (main-C) | The carbonyl group stabilises the carbocation intermediates. | electrostatic stabiliser, polar interaction |
| Thr403 | Thr403A | The hydroxyl group stabilises the carbocation intermediates. | electrostatic stabiliser, polar interaction |
| Asp444 | Asp444A | Acts as a general acid and base, removing the hydroxyl proton from Tyr 520 and after the protonation of germacrede C6, redonating the proton back to Tyr 520. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| Tyr520 | Tyr520A | Donates a proton, accepted from Asp 525, to germacrene C6 in the formation of the eudesmane carbocation intermediate. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, proton relay |
| Asp525 | Asp525A | Abstracts a proton from C13 to promote the formation of the germacrene intermediate. It then donates the proton to Tyr 520 to allow it to act as an acid. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| Tyr527 | Tyr527A | The quadrupole of Tyr 527 stabilises the positive charge on C11, created after the formation of the C1-C10 bond. | polar interaction, electrostatic stabiliser |
| Arg441, Arg264 | Arg441A, Arg264A | Stabilises the additional negative charge on the departing diphosphate in the first step of the reaction. | promote heterolysis, hydrogen bond donor, electrostatic stabiliser |
| Trp273 | Trp273A | The aromatic quadruple of Trp273 stabilises the electrostatic centre of eudesmane carbocation at C7. It abstracts a proton from C8, forming the final product, 5-epi-aristolochene. | hydrogen bond donor, proton acceptor, proton donor, polar interaction, electrostatic stabiliser |
Chemical Components
heterolysis, charge delocalisation, dephosphorylation, intermediate formation, overall reactant used, overall product formed, intramolecular electrophilic addition, cyclisation, proton transfer, proton relay, hydride transfer, intramolecular rearrangement, intermediate terminated, inferred reaction step, native state of enzyme regeneratedReferences
- Starks CM et al. (1997), Science, 277, 1815-1820. Structural Basis for Cyclic Terpene Biosynthesis by Tobacco 5-Epi-Aristolochene Synthase. DOI:10.1126/science.277.5333.1815. PMID:9295271.
- Faraldos JA et al. (2007), Chembiochem, 8, 1826-1833. Interception of the enzymatic conversion of farnesyl diphosphate to 5-epi-aristolochene by using a fluoro substrate analogue: 1-fluorogermacrene A from (2E,6Z)-6-fluorofarnesyl diphosphate. DOI:10.1002/cbic.200700398. PMID:17886322.

Step 1. The substrate undergoes heterolysis. The diphosphate product remains associated with the active site, and the cabocation is delocalised over the three terminal carbon atoms of the intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, promote heterolysis, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, promote heterolysis, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Thr401A (main-C) | electrostatic stabiliser, polar interaction |
| Thr402A (main-C) | electrostatic stabiliser, polar interaction |
| Thr403A | electrostatic stabiliser, polar interaction |
Chemical Components
heterolysis, charge delocalisation, dephosphorylation, intermediate formation, overall reactant used, overall product formed
Step 2. The C10-C11 double bond initiates an electrophilic attack on the positive carbocation, forming the first cyclic intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Tyr527A | polar interaction, electrostatic stabiliser |
| Thr401A (main-C) | electrostatic stabiliser, polar interaction |
| Thr402A (main-C) | electrostatic stabiliser, polar interaction |
| Thr403A | electrostatic stabiliser, polar interaction |
Chemical Components
ingold: intramolecular electrophilic addition, charge delocalisation, cyclisation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Asp525A | hydrogen bond acceptor |
| Tyr527A | polar interaction, electrostatic stabiliser |
| Thr401A (main-C) | electrostatic stabiliser, polar interaction |
| Thr402A (main-C) | electrostatic stabiliser, polar interaction |
| Thr403A | electrostatic stabiliser, polar interaction |
| Asp525A | proton acceptor |
Chemical Components
proton transfer, intermediate formation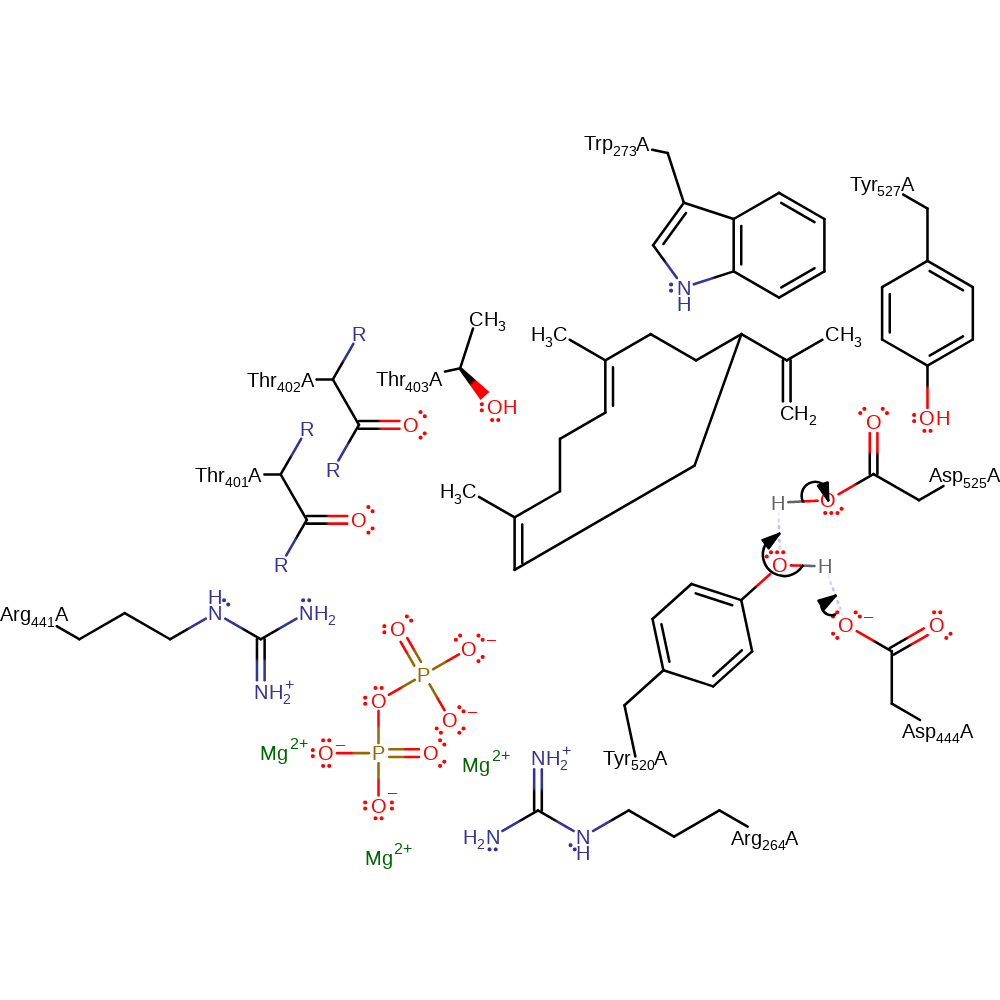
Step 4. Asp444 in the Asp444-Tyr520-Asp525 triad deprotonated Tyr520, which in turn deprotonates Asp525.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor, hydrogen bond acceptor, proton relay |
| Asp444A | hydrogen bond acceptor |
| Asp525A | hydrogen bond donor |
| Asp444A | proton acceptor |
| Tyr520A | proton acceptor |
| Asp525A | proton donor |
| Tyr520A | proton donor |
Chemical Components
proton transfer, proton relay
Step 5. The C2-C3 double bond initiates an electrophilic addition to the C6 double bond in an anti-Markovnikov cyclisation, which deprotonates Tyr520, in turn deprotonating Asp444.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor, hydrogen bond acceptor, proton relay |
| Asp444A | hydrogen bond donor |
| Thr401A (main-C) | polar interaction, electrostatic stabiliser |
| Thr402A (main-C) | polar interaction, electrostatic stabiliser |
| Thr403A | polar interaction, electrostatic stabiliser |
| Tyr520A | proton acceptor |
| Asp444A | proton donor |
| Tyr520A | proton donor |
Chemical Components
ingold: intramolecular electrophilic addition, proton transfer, cyclisation, intermediate formation, proton relay
Step 6. The C2 hydride migrates to the planar C3 carbocation along the top of the bicyclic ring system leaving a planar tertiary carbocation at C2.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Thr401A (main-C) | polar interaction, electrostatic stabiliser |
| Thr402A (main-C) | polar interaction, electrostatic stabiliser |
| Thr403A | polar interaction, electrostatic stabiliser |
Chemical Components
hydride transfer, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Trp273A | polar interaction, electrostatic stabiliser |
| Thr401A (main-C) | polar interaction, electrostatic stabiliser |
| Thr402A (main-C) | polar interaction, electrostatic stabiliser |
| Thr403A | polar interaction, electrostatic stabiliser |
Chemical Components
intramolecular rearrangement, intermediate formation
Step 8. Trp273 has been proposed as the base in this step as the size and positioning of the indole ring allow it to accept the acidic proton from the intermediate. Further, the greatly reduced nucleophilicity of the indole ring greatly diminishes the likelihood of inadvertant enzyme alkylation by the highly reactive carbocation intermediate. The authors note that there is chemical precedence for both quadrupole mediated cation interactions and for electrophilic aromatic substuitutions iin idole ring systems. The arenium cation formed upon protonation of teh indole ring is more energetically stable than the eudesmane carbocation intermediates due to the presence of the nitrogen atom and the extended conjugation of the indole ring [PMID:9295271].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Trp273A | polar interaction, electrostatic stabiliser |
| Trp273A | proton acceptor |
Chemical Components
proton transfer, intermediate terminated, overall product formed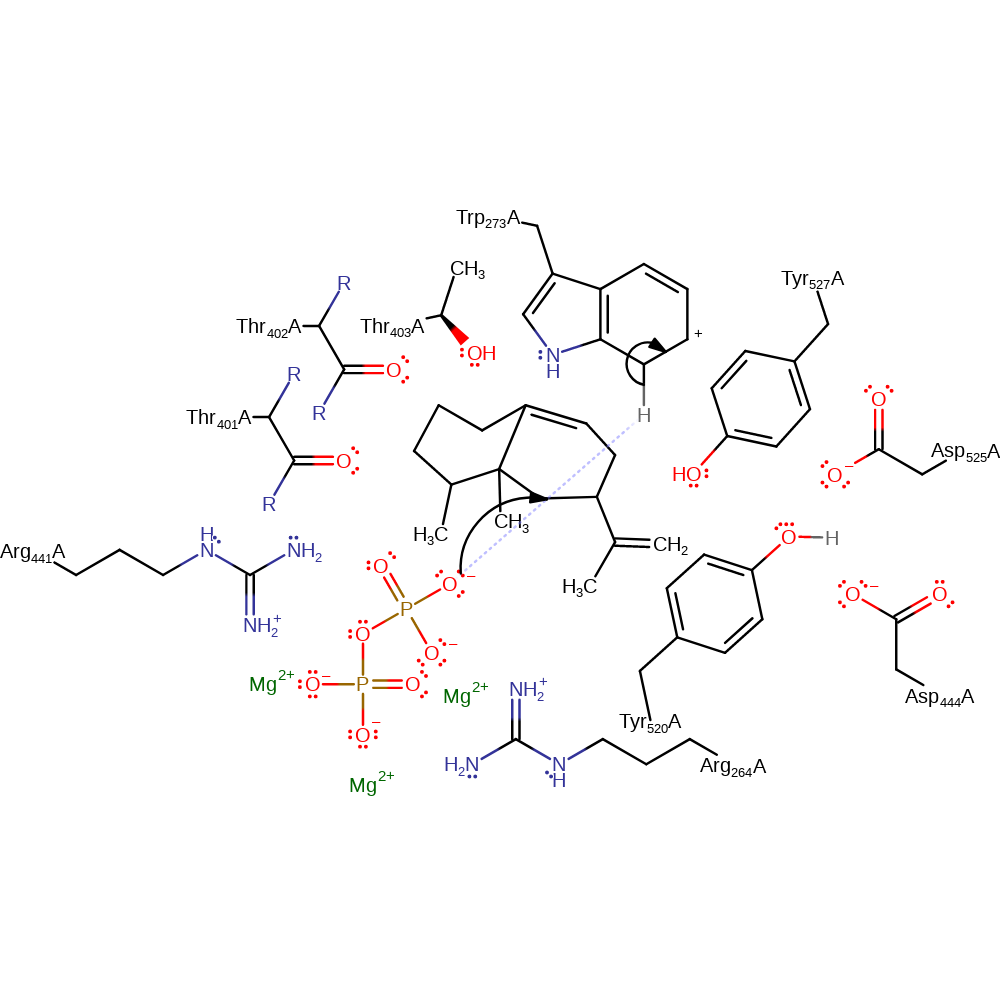
Step 9. Trp273 is deprotonated in order to renegerate the active state of the enzyme.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg441A | electrostatic stabiliser, hydrogen bond donor |
| Arg264A | electrostatic stabiliser, hydrogen bond donor |
| Tyr520A | hydrogen bond donor |
| Asp444A | hydrogen bond acceptor |
| Trp273A | hydrogen bond donor |
| Trp273A | proton donor |
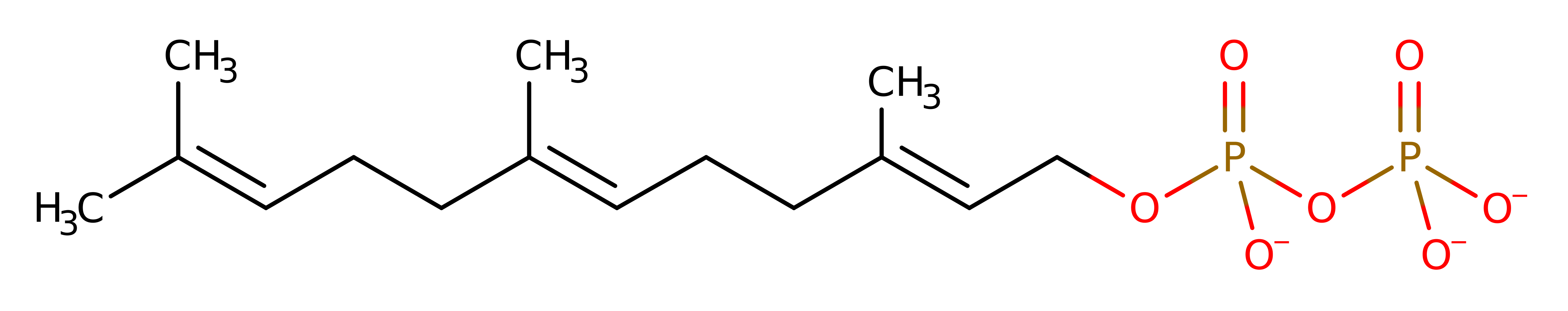

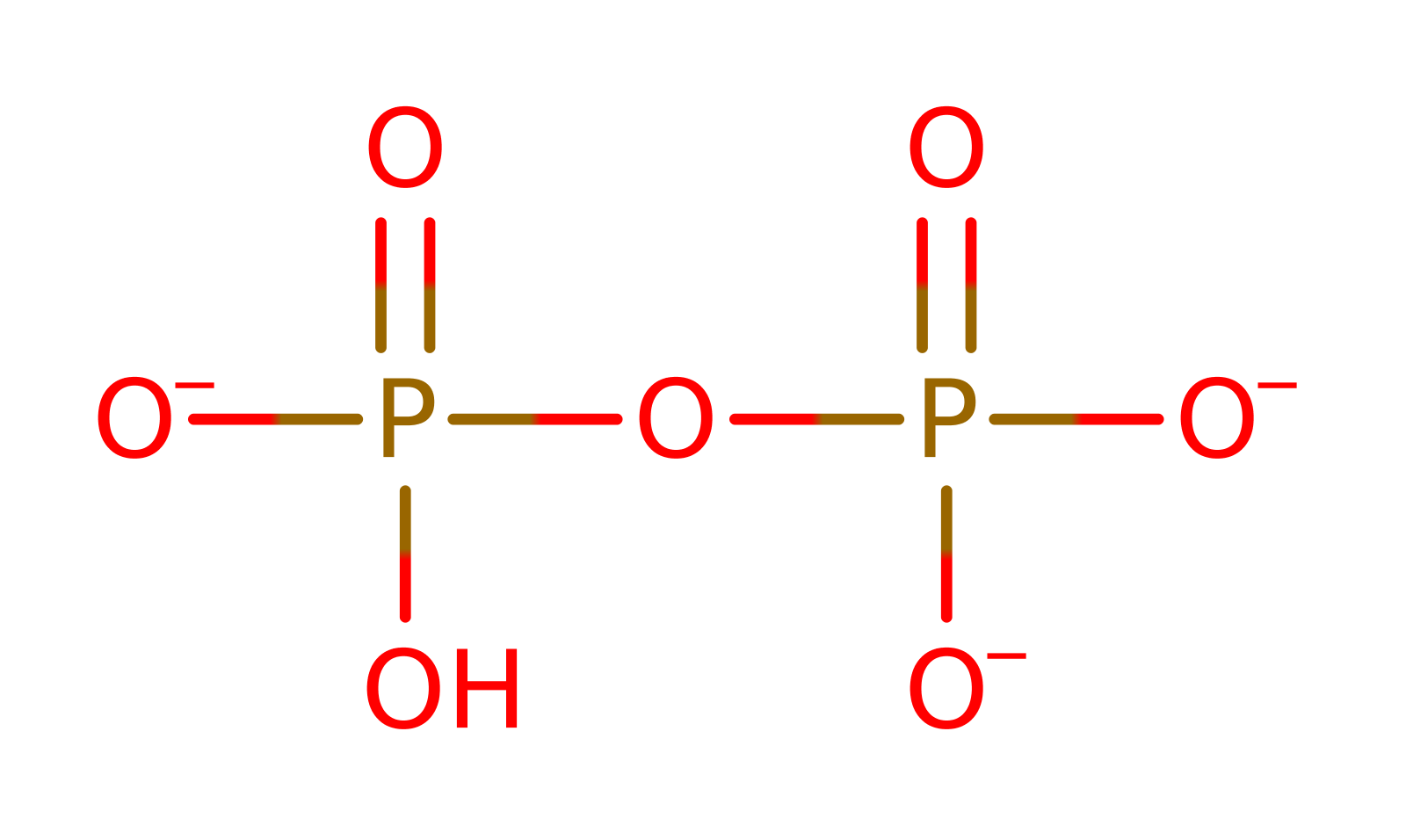

 Download:
Download: