Pyruvate oxidase
Pyruvate oxidase requires both thiamine diphosphate and FAD as cofactors. It catalyses the decarboxylation of pyruvate in four steps and the energy released it partially stored in acetyl phosphate. It is important for aerobic growth.
Reference Protein and Structure
- Sequence
-
P37063
 (1.2.3.3)
(1.2.3.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Lactobacillus plantarum WCFS1 (Lactobacillus plantarum)

- PDB
-
1pow
- THE REFINED STRUCTURES OF A STABILIZED MUTANT AND OF WILD-TYPE PYRUVATE OXIDASE FROM LACTOBACILLUS PLANTARUM
(2.5 Å)



- Catalytic CATH Domains
-
3.40.50.1220
 3.40.50.970
3.40.50.970  (see all for 1pow)
(see all for 1pow)
- Cofactors
- Fadh2(2-) (1), Thiamine(1+) diphosphate(3-) (1), Magnesium(2+) (1) Metal MACiE
Enzyme Reaction (EC:1.2.3.3)
Enzyme Mechanism
- Summary
- Step 1
- Step 2
- Step 3
- Step 4
- Step 5
- Step 6
- Step 7
- Step 8
- Step 9
- Step 10
- Step 11
- Step 12
- Products
- All Steps
Introduction
The TPP (thiamine diphosphate) cofactor is activated by the formation of an ionic N1'-H...O bond between the N1' atom of the aminopyrimidine ring of the cofactor and the OG of the intrinsic Glu59, resulting in the transfer of a negative charge from the protein to the cofactor. This transfer then imposes an active V-conformation that brings the N4' atom of the cofactor to the distance required for the intramolecular C2-H...N4' hydrogen bond with the thiazolium C2 atom. This initial activation is then followed by abstraction of the proton from the C2 atom. The resulting carbanion of thiamine diphosphate initiates a nucleophilic attack on the carbonyl carbon of pyruvate in an addition reaction. Pyruvate is now covalently attached to the TPP cofactor and undergoes decarboxylation. This is followed by a single electron transfer from the high energy thamine diphosphate enamine intermediate to the FAD, resulting in bond order rearrangement and deprotonation of the alcohol group present on the intermediate. This intermediate undergoes tautomerisatoin and the thiamine ring nitrogen acts as an electron sink in the formation of the radical tautomer. Phosphate initiates a nucleophilic attack on the kinetically stable anion radical adduct. The high energy phosphate radical delivers a second reducing equivalent to the FAD semiquinone. The tetrahedral anion intermediate collapses. This forms the high energy metabolite acetyl-phosphate. The FAD diradical transfers one of its electrons to dioxygen with subsequent loss of a single proton. The FADH transfers the second radical and proton to the dioxygen to regenerate the FAD cofactor and hydrogen peroxide. The TTP cofactor is regenerated by reprotonation of the C2 position.
Catalytic Residues Roles
| UniProt | PDB* (1pow) | ||
| Phe479, Ile480 | Phe479(471)A, Ile480(472)A | The non-polar environment created by the presence of lle480 and Phe479 acts to raise the energy of the adduct through polar-non polar interactions. This lowers the activation barrier towards decarboxylation. The envirionment also helps stabilise the radical intermediates produced. | radical stabiliser, promote heterolysis, electrostatic destabiliser, polar/non-polar interaction |
| Glu483, Arg264 | Glu483(475)A, Arg264(256)A | Help stabilise the residues through which the electrons are proposed to pass. | radical stabiliser |
| Val394, Gln122 | Val394(386)A, Gln122(114)A(AA) | The steric and electrostatic interactions between the intermediate and residues Val394 and Gln122, respectively holds the TPP cofactor in a high energy conformation which also contributes to enhanced reactivity. | promote heterolysis, radical stabiliser, steric role, polar/non-polar interaction |
| Phe121 | Phe121(113)A(AA) | Helps stabilise the reactive radical intermediates formed during the course of the reaction. It is also possible that this residue helps transfer the electrons from the TPP cofactor to FAD. | radical stabiliser, promote heterolysis, electrostatic destabiliser, polar/non-polar interaction |
| Glu59 | Glu59(51)A(AA) | Acts as a general acid/base in the activation of the thiamine diphosphate cofactor. | hydrogen bond acceptor, proton acceptor, proton donor, activator, increase acidity |
Chemical Components
proton transfer, cofactor used, intermediate formation, bimolecular nucleophilic addition, unimolecular elimination by the conjugate base, overall product formed, decarboxylation, intermediate collapse, electron transfer, radical formation, rate-determining step, tautomerisation (not keto-enol), inferred reaction step, bimolecular elimination, redox reaction, overall reactant used, intermediate terminated, native state of cofactor regenerated, native state of enzyme regeneratedReferences
- Muller YA et al. (1993), Science, 259, 965-967. Structure of the thiamine- and flavin-dependent enzyme pyruvate oxidase. DOI:10.1126/science.8438155. PMID:8438155.
- Tittmann K (2009), FEBS J, 276, 2454-2468. Reaction mechanisms of thiamin diphosphate enzymes: redox reactions. DOI:10.1111/j.1742-4658.2009.06966.x. PMID:19476487.
- Wille G et al. (2006), Nat Chem Biol, 2, 324-328. The catalytic cycle of a thiamin diphosphate enzyme examined by cryocrystallography. DOI:10.1038/nchembio788. PMID:16680160.
- Wille G et al. (2005), Biochemistry, 44, 5086-5094. The Role of Val-265 for Flavin Adenine Dinucleotide (FAD) Binding in Pyruvate Oxidase: FTIR, Kinetic, and Crystallographic Studies on the Enzyme Variant V265A†,‡. DOI:10.1021/bi047337o. PMID:15794646.
- Tittmann K et al. (2000), Biochemistry, 39, 10747-10754. Mechanism of Elementary Catalytic Steps of Pyruvate Oxidase fromLactobacillus plantarum†. DOI:10.1021/bi0004089. PMID:10978159.
- Tittmann K et al. (1998), J Biol Chem, 273, 12929-12934. Activation of Thiamin Diphosphate and FAD in the Phosphatedependent Pyruvate Oxidase fromLactobacillus plantarum. DOI:10.1074/jbc.273.21.12929. PMID:9582325.
- Muller YA et al. (1994), J Mol Biol, 237, 315-335. The Refined Structures of a Stabilized Mutant and of Wild-type Pyruvate Oxidase from Lactobacillus plantarum. DOI:10.1006/jmbi.1994.1233. PMID:8145244.

Step 1. The TTP cofactor adopts the V configuration which brings the 4 imino group of the aminopyrimidine ring close to the C2 carbon of the thiazolium ring [PMID:16680160]. Hydrogen bonding of the carboxylate group from Glu59' to N1' of the aminopyrimidine ring increases the basic nature of the 4' position by stabilising the 4'-imino tautomeric form, allowing the group to deprotonate the C1 position of the thiamine ring. As a result, the cofactor is activated towards electrophilic attack.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor, activator, increase acidity |
| Glu59(51)A(AA) | proton acceptor |
Chemical Components
proton transfer, cofactor used, intermediate formation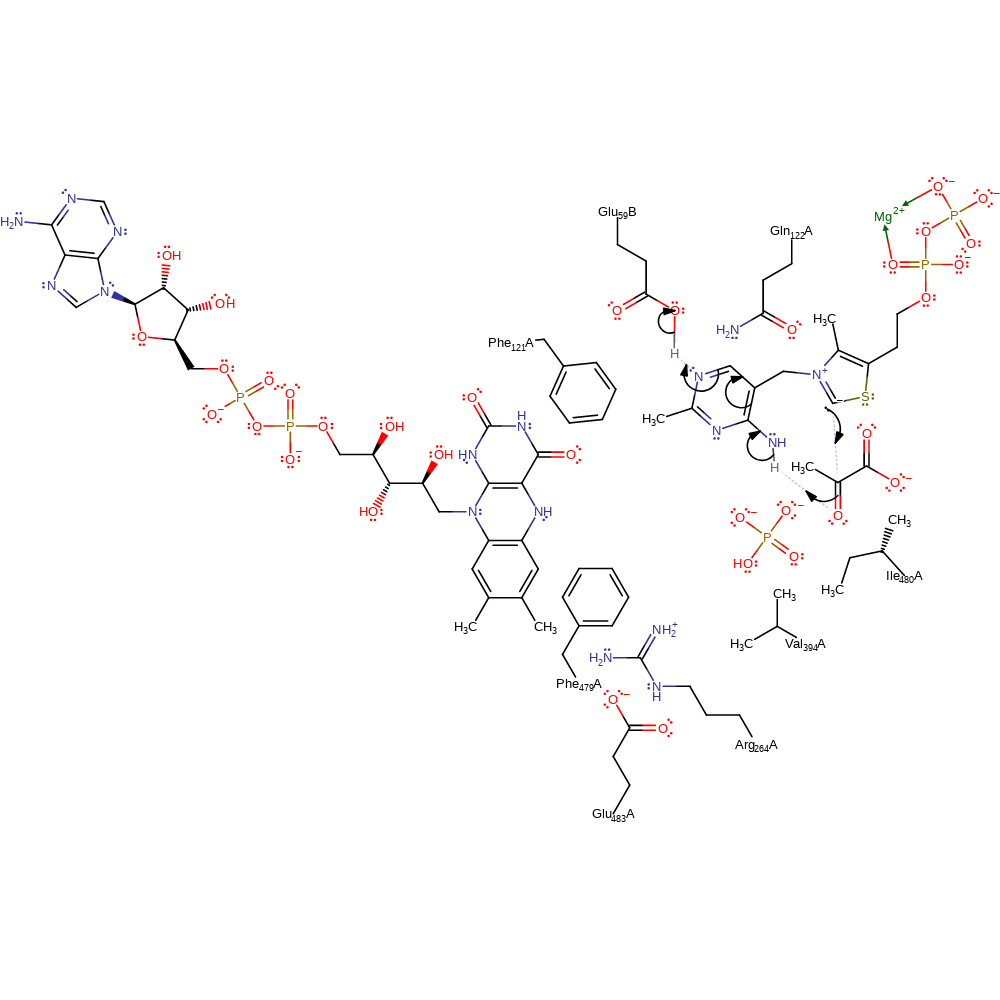
Step 2. The carbanion of thiamine diphosphate initiates a nucleophilic attack on the carbonyl carbon of pyruvate in an addition reaction. The conjugated double bond system of the cofactor undergoes rearrangement which results in the deprotonation of Glu59'. The 4' enamine tautomer is favoured by the formation of a strong hydrogen bond between the side chain carbonyl group of Glu59' and the N1 hydrogen.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | activator, increase acidity, hydrogen bond acceptor |
| Gln122(114)A(AA) | activator, hydrogen bond acceptor |
| Glu59(51)A(AA) | proton donor |
Chemical Components
ingold: bimolecular nucleophilic addition, proton transfer, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | steric role, promote heterolysis, polar/non-polar interaction |
| Ile480(472)A | electrostatic destabiliser, promote heterolysis, polar/non-polar interaction |
| Phe121(113)A(AA) | electrostatic destabiliser, promote heterolysis, polar/non-polar interaction |
| Phe479(471)A | electrostatic destabiliser, promote heterolysis, polar/non-polar interaction |
| Gln122(114)A(AA) | electrostatic destabiliser, promote heterolysis, hydrogen bond donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, overall product formed, decarboxylation, intermediate collapse, intermediate formation
Step 4. A single electron is transferred from the high energy thamine diphosphate enamine intermediate to the FAD, resulting in bond order rearrangement and deprotonation of the alcohol group present on the intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
| Arg264(256)A | radical stabiliser |
| Glu483(475)A | radical stabiliser |
Chemical Components
electron transfer, proton transfer, radical formation, cofactor used, intermediate formation, rate-determining stepCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
tautomerisation (not keto-enol), inferred reaction step, intermediate formation
Step 6. The thiamine ring nitrogen acts as an electron sink in the formation of the radical tautomer.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
tautomerisation (not keto-enol)
Step 7. Phosphate attacks the kinetically stable anion radical adduct. Kinetics experiments have shown the presence of phosphate to enhance the rate of electron transfer [PMID:19476487]. The negative charge resulting from the nucleophilic attack by phosphate is thought to reduce the potential of the intermediate and therefore increase the driving force of the following electron transfer to FAD.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
ingold: bimolecular nucleophilic addition
Step 8. The high energy phosphate radical delivers a second reducing equivalent to the FAD semiquinone.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Arg264(256)A | radical stabiliser |
| Glu483(475)A | radical stabiliser |
Chemical Components
electron transfer, proton transfer, intermediate formation
Step 9. Prior to this step there is a rearrangement of electrons in the TPP-substrate adduct which results in a negative charge on the oxygen atom. The tetrahedral anion intermediate collapses. This forms the high energy metabolite acetyl-phosphate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, overall product formed
Step 10. The FAD diradical transfers one of its electrons to dioxygen with subsequent loss of a single proton.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
electron transfer, ingold: bimolecular elimination, redox reaction, overall reactant used, intermediate formation
Step 11. The FADH transfers the second radical and proton to the dioxygen to regenerate the FAD cofactor and hydrogen peroxide.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Val394(386)A | radical stabiliser |
| Ile480(472)A | radical stabiliser |
| Phe121(113)A(AA) | radical stabiliser |
| Phe479(471)A | radical stabiliser |
| Gln122(114)A(AA) | hydrogen bond donor |
Chemical Components
electron transfer, proton transfer, intermediate terminated, native state of cofactor regenerated, overall product formed
Step 12. The C2 atom of the thamine ring is exposed to the solvent. Reprotonation of the anion regenerates the TPP cofactor.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu59(51)A(AA) | hydrogen bond acceptor |
| Gln122(114)A(AA) | hydrogen bond donor |









 Download:
Download: