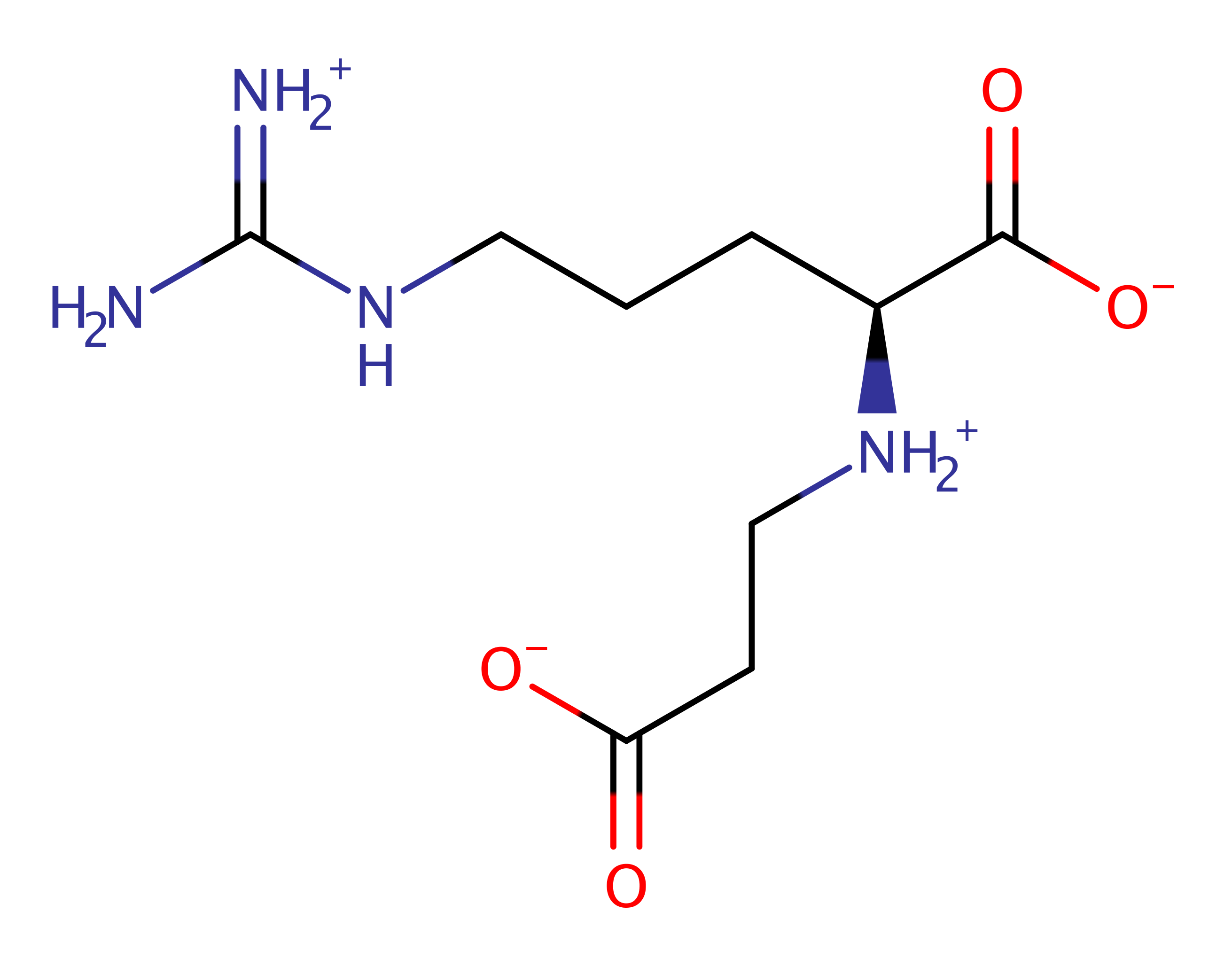
(carboxyethyl)arginine beta-lactam-synthase
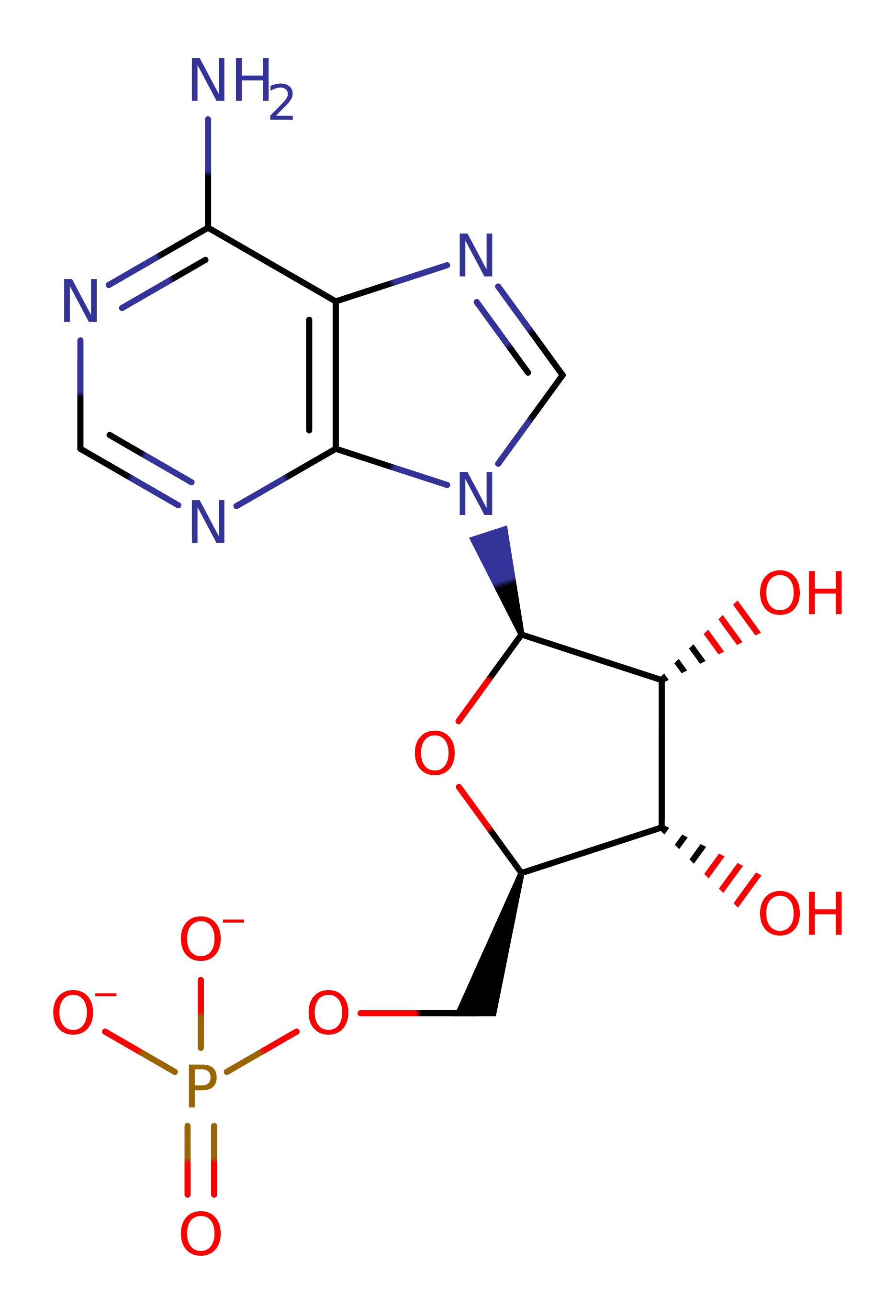
Asparagine synthetase B catalyses the ATP-dependent conversion of aspartate to asparagine [PMID: 11551215]. This enzyme is a homodimer, with each monomer composed of a glutaminase domain and a synthetase domain. It forms part of the pathway for the biosythesis of the beta-lactamase inhibitor clavulanate in Streptomyces clavuligerus. This entry represents the reaction occuring in the synthetase domain in which the L-N2-(2-carboxyethyl)arginine is first converted into an acyl-AMP by reaction with ATP and loss of diphosphate, and that the beta-lactam ring is then formed by the intramolecular attack of the beta-nitrogen on the activated carboxy group [PMID:9689037].
Reference Protein and Structure
- Sequence
-
P0DJQ7
 (6.3.3.4)
(6.3.3.4)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Streptomyces clavuligerus (Bacteria)

- PDB
-
1mb9
- BETA-LACTAM SYNTHETASE COMPLEXED WITH ATP
(2.11 Å)



- Catalytic CATH Domains
-
3.40.50.620
 (see all for 1mb9)
(see all for 1mb9)
- Cofactors
- Magnesium(2+) (2) Metal MACiE
Enzyme Reaction (EC:6.3.3.4)
Enzyme Mechanism
Introduction
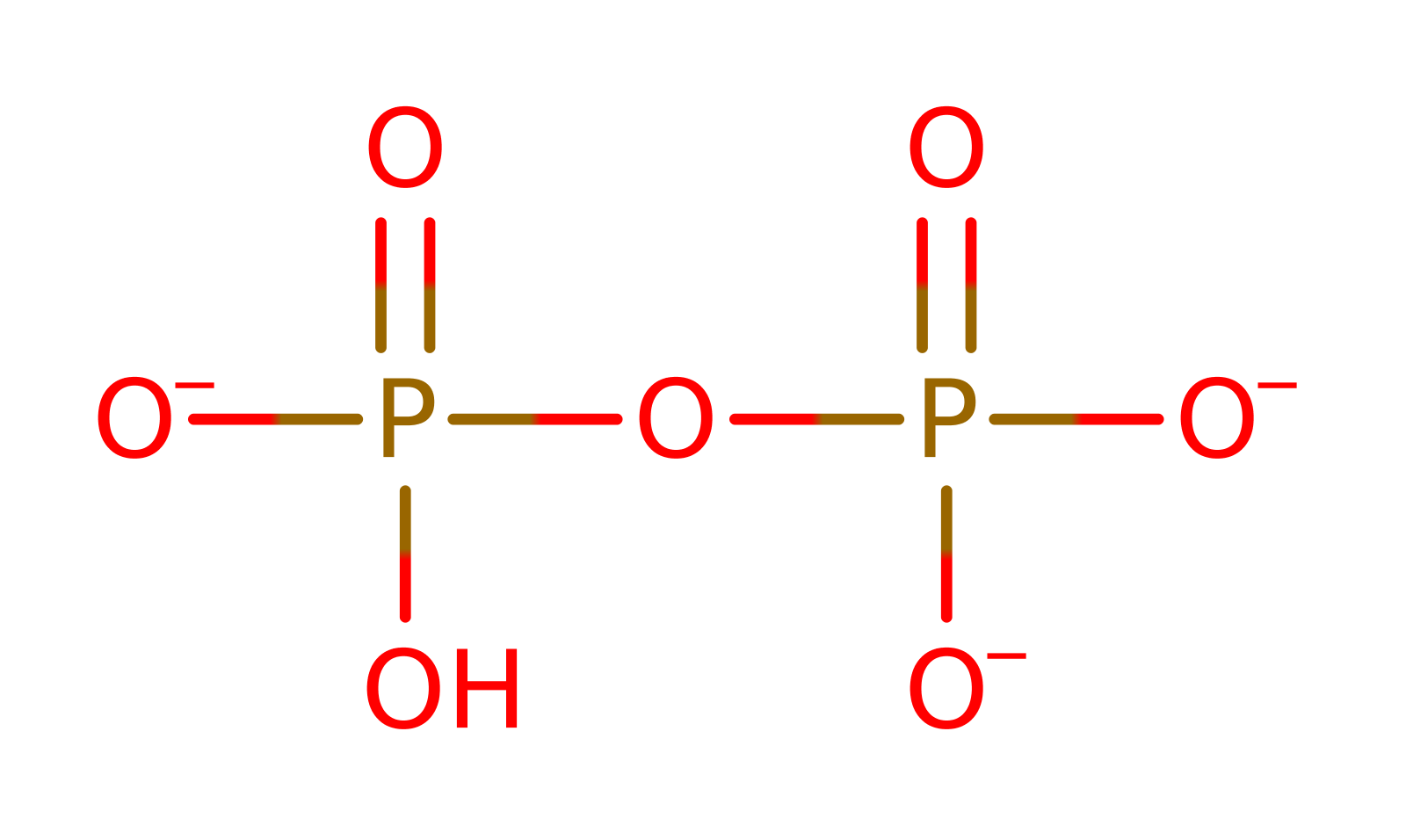
The L-N2-(2-carboxyethyl)arginine substrate initiates a nucleophilic attack on the alpha-phosphate of ATP in a substitution reaction. The beta-lactam ring is then formed by the intramolecular attack of the beta-nitrogen on the activated carboxy group. It is worth noting that in this protein the Gly-Tyr dyad is actually in the reverse of the "normal" protonation state, i.e. Glu is neutral and Tyr is negatively charged [PMID:19371088].
Catalytic Residues Roles
| UniProt | PDB* (1mb9) | ||
| Glu382 | Glu382A | This residue is neutral in the enzyme's ground state. Helps activate the catalytic tyrosine. | increase basicity, hydrogen bond donor, electrostatic stabiliser, increase acidity |
| Tyr348 | Tyr348A | Acts as a general acid/base. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor |
| Lys443 | Lys443A | Helps stabilise the reactive intermediates formed during the course of the reaction. | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
bimolecular nucleophilic substitution, overall reactant used, intermediate formation, overall product formed, proton transfer, intermediate collapse, cyclisation, inferred reaction step, native state of enzyme regeneratedReferences
- Miller MT et al. (2002), Proc Natl Acad Sci U S A, 99, 14752-14757. The catalytic cycle of -lactam synthetase observed by x-ray crystallographic snapshots. DOI:10.1073/pnas.232361199. PMID:12409610.
- Labonte JW et al. (2012), Medchemcomm, 3, 960-966. Engineering the synthetic potential of β-lactam synthetase and the importance of catalytic loop dynamics. DOI:10.1039/c2md00305h. PMID:23616913.
- Raber ML et al. (2009), Chembiochem, 10, 2904-2912. A Conserved Lysine in β-Lactam Synthetase Assists Ring Cyclization: Implications for Clavam and Carbapenem Biosynthesis. DOI:10.1002/cbic.200900389. PMID:19882698.
- Raber ML et al. (2009), Biochemistry, 48, 4959-4971. A Conserved Tyrosyl−Glutamyl Catalytic Dyad in Evolutionarily Linked Enzymes: Carbapenam Synthetase and β-Lactam Synthetase. DOI:10.1021/bi900432n. PMID:19371088.

Step 1. The L-N2-(2-carboxyethyl)arginine substrate initiates a nucleophilic attack on the alphs-phosphate of ATP in a substitution reaction.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr348A | hydrogen bond acceptor |
| Glu382A | hydrogen bond donor, electrostatic stabiliser |
| Lys443A | hydrogen bond donor |
Chemical Components
ingold: bimolecular nucleophilic substitution, overall reactant used, intermediate formation, overall product formed
Step 2. Tyr348 deprotonates the immine, which initiates a nucleophilic attack on the carbonyl carbon next to the phosphate group in a substitution reaction. The leaving phosphate group deprotonates the newly formed beta-lactam. It is thought that this is a concerted mechanism which proceeds via a tetrahedral transition state, instead of the stabilised intermediate [PMID:12409610].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr348A | hydrogen bond acceptor |
| Glu382A | hydrogen bond donor, increase basicity, electrostatic stabiliser |
| Lys443A | hydrogen bond donor, electrostatic stabiliser |
| Tyr348A | proton acceptor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic substitution, overall product formed, intermediate collapse, cyclisationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr348A | hydrogen bond donor |
| Glu382A | hydrogen bond donor, increase acidity, electrostatic stabiliser |
| Tyr348A | proton donor |




 Download:
Download: