Naringenin-chalcone synthase
Chalcone synthase (CHS) is pivotal for the biosynthesis of flavonoid antimicrobial phytoalexins and anthocyanin pigments in plants. It produces chalcone by condensing one p-coumaroyl- and three malonyl-coenzyme A thioesters into a polyketide reaction intermediate that cyclises without the involvement of specific catalytic residues.
Reference Protein and Structure
- Sequence
-
P30074
 (2.3.1.74)
(2.3.1.74)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Medicago sativa (Alfalfa)

- PDB
-
1cgk
- CHALCONE SYNTHASE FROM ALFALFA COMPLEXED WITH NARINGENIN
(1.84 Å)



- Catalytic CATH Domains
-
3.40.47.10
 (see all for 1cgk)
(see all for 1cgk)
Enzyme Reaction (EC:2.3.1.74)
Enzyme Mechanism
- Summary
- Step 1
- Step 2
- Step 3
- Step 4
- Step 5
- Step 6
- Step 7
- Step 8
- Step 9
- Step 10
- Step 11
- Step 12
- Step 13
- Step 14
- Step 15
- Step 16
- Step 17
- Step 18
- Step 19
- Step 20
- Products
- All Steps
Introduction
Binding of p-coumaroyl-CoA initiates the reaction. The thiolate of Cys164 attacks the thioester carbonyl, resulting in transfer of the coumaroyl moiety to the cysteine side chain. Asn336 hydrogen bonds with the thioester carbonyl, further stabilizing formation of the tetrahedral reaction intermediate. CoA then dissociates from the enzyme, leaving a coumaroyl-thioester at Cys164. Malonyl-CoA binds and positions the bridging carbon of the malonyl moiety near the carbonyl of the enzyme-bound coumaroyl thioester. Asn336 orients the thioester carbonyl of malonyl-CoA near His303 with Phe215, providing a nonpolar environment for the terminal carboxylate that facilitates decarboxylation. His303 and Asn336 interact with the substrate's thioester carbonyl, creating an efficient oxyanion hole that stabilizes the developing negative charge during the decarboxylation step through stabilization of the enol tautomer of the acetyl anion. Attack of the carbanion on the carbonyl of the enzyme-bound coumaroyl thioester releases the thiolate anion of Cys164 and transfers the coumaroyl group to the acetyl moiety of the CoA thioester. Hydrogen bonds from His303 and Asn336 would stabilize the tetrahedral transition state of this reaction. Recapture of the elongated coumaroyl-acetyl-diketide-CoA by Cys164 and release of CoA set the stage for two additional rounds of elongation, resulting in formation of the final tetraketide reaction intermediate. The final step in chalcone formation involves an intramolecular Claisen condensation encompassing the three acetate units derived from three malonyl-CoAs. During cyclization, the nucleophilic methylene group nearest the coumaroyl moiety attacks the carbonyl carbon of the thioester linked to Cys164. Ring closure is proposed to proceed through an internal proton transfer from the nucleophilic carbon to the carbonyl oxygen. Breakdown of this tetrahedral intermediate expels the newly cyclized ring system from Cys164. Subsequent aromatization of the trione ring through a second series of facile internal proton transfers yields chalcone.
Catalytic Residues Roles
| UniProt | PDB* (1cgk) | ||
| Phe215 | Phe215A | Provides a non-polar environment which encourages the formation of CO2 during decarboxylation. | electrostatic destabiliser, polar/non-polar interaction |
| His303 | His303A | The protonated residue forms a stable thiolate-imidazolium ion pair thus activating Cys164. This group also forms part of an oxyanion hole during decarboxylation. | hydrogen bond donor, electrostatic stabiliser |
| Cys164 | Cys164A | Serves as the nucleophile for polyketide formation, forming a enzyme adduct and releasing CoA. | activator, covalently attached, nucleofuge, nucleophile |
| Asn336 | Asn336A | Forms part of an oxyanion hole during decarboxylation. | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
bimolecular nucleophilic addition, overall reactant used, intermediate formation, enzyme-substrate complex formation, elimination (not covered by the Ingold mechanisms), intermediate collapse, overall product formed, intramolecular elimination, decarboxylation, enzyme-substrate complex cleavage, intramolecular nucleophilic addition, proton transfer, cyclisation, inferred reaction step, native state of enzyme regenerated, intramolecular rearrangementReferences
- Jez JM et al. (2000), Biochemistry, 39, 890-902. Dissection of Malonyl-Coenzyme A Decarboxylation from Polyketide Formation in the Reaction Mechanism of a Plant Polyketide Synthase†. DOI:10.1021/bi991489f. PMID:10653632.
- Healy EF et al. (2018), Biochem Biophys Res Commun, 497, 1123-1128. A unified mechanism for plant polyketide biosynthesis derived from in silico modeling. DOI:10.1016/j.bbrc.2018.02.190. PMID:29496450.
- Li D et al. (2009), Theor Chem Acc, 122, 157-166. A quantum mechanics study on the reaction mechanism of chalcone formation from p-coumaroyl-CoA and malonyl-CoA catalyzed by chalcone synthase. DOI:10.1007/s00214-008-0495-7.
- Abe I et al. (2000), J Am Chem Soc, 122, 11242-11243. Substrate Specificity of Chalcone Synthase: Enzymatic Formation of Unnatural Polyketides from Synthetic Cinnamoyl-CoA Analogues. DOI:10.1021/ja0027113.
- Ferrer JL et al. (1999), Nat Struct Biol, 6, 775-784. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. DOI:10.1038/11553. PMID:10426957.
- Tropf S et al. (1995), J Biol Chem, 270, 7922-7928. Reaction mechanisms of homodimeric plant polyketide synthase (stilbenes and chalcone synthase). A single active site for the condensing reaction is sufficient for synthesis of stilbenes, chalcones, and 6'-deoxychalcones. PMID:7713888.

Step 1. The anionic Cys164 attacks at the 4-coumaroyl-CoA thio-carbonyl, forming an anionic tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | electrostatic stabiliser, hydrogen bond donor |
| Cys164A | activator, nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, intermediate formation, enzyme-substrate complex formation
Step 2. The tetrahedral intermediate collapses, eliminating coenzyme A and foming a covalently bonded substrate-enzyme intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | electrostatic stabiliser, hydrogen bond donor |
| Cys164A | activator, covalently attached |
| Phe215A | polar/non-polar interaction, electrostatic destabiliser |
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate collapse, overall product formed
Step 3. The polar-nonpolar interaction of Phe215 and malonyl-CoA drives decarboxylation to form the enolate intermediate which is stabilised through hydrogen bonding with Asn336.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
| Phe215A | polar/non-polar interaction, electrostatic destabiliser |
Chemical Components
ingold: intramolecular elimination, intermediate formation, overall reactant used, decarboxylation, overall product formed
Step 4. The enolate intermediate attacks the thio-carbonyl, forming an anionic tetrahedral intermediate while extending the carbon chain by two units.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
Chemical Components
ingold: bimolecular nucleophilic addition, intermediate formation
Step 5. The tetrahedral intermediate collapses, eliminating the Cys164 sidechain. The position of Phe215 postulates its involvement in driving the decomposition of the tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator |
| Phe215A | polar/non-polar interaction |
| Cys164A | nucleofuge |
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate formation, enzyme-substrate complex cleavage
Step 6. Cys164 attacks at the thio-carbonyl, reforming an enzyme-substrate tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, intermediate formation, enzyme-substrate complex formation
Step 7. The tetrahedral intermediate collapses, eliminating coenzyme A and forming the enzyme-bonded mono-ketide product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate formation, overall product formed
Step 8. The polar-nonpolar interaction of Phe215 and malonyl-CoA drives decarboxylation to form the enolate intermediate which is stabilised through hydrogen bonding with Asn336.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
| Phe215A | polar/non-polar interaction, electrostatic destabiliser |
Chemical Components
ingold: intramolecular elimination, intermediate formation, overall reactant used, decarboxylation, overall product formed
Step 9. The enolate intermediate attacks the thio-carbonyl, forming an anionic tetrahedral intermediate while extending the carbon chain by two units.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
Chemical Components
ingold: bimolecular nucleophilic addition, intermediate formation
Step 10. The tetrahedral intermediate collapses, eliminating the Cys164 sidechain. The position of Phe215 postulates its involvement in driving the decomposition of the tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator |
| Phe215A | polar/non-polar interaction |
| Cys164A | nucleofuge |
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate formation, enzyme-substrate complex cleavage
Step 11. Cys164 attacks at the thio-carbonyl, reforming an enzyme-substrate tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, intermediate formation, enzyme-substrate complex formation
Step 12. The tetrahedral intermediate collapses, eliminating coenzyme A and forming the enzyme-bonded mono-ketide product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
Chemical Components
elimination (not covered by the Ingold mechanisms), intermediate formation, overall product formed
Step 13. The polar-nonpolar interaction of Phe215 and malonyl-CoA drives decarboxylation to form the enolate intermediate which is stabilised through hydrogen bonding with Asn336.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Phe215A | electrostatic destabiliser, polar/non-polar interaction |
| Cys164A | covalently attached, activator |
| His303A | hydrogen bond donor |
| Asn336A | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
overall product formed, decarboxylation, overall reactant used, intermediate formation, ingold: intramolecular elimination
Step 14. The enolate intermediate attacks the thio-carbonyl, forming an anionic tetrahedral intermediate while extending the carbon chain by two units.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys164A | covalently attached, activator |
| His303A | hydrogen bond donor |
| Asn336A | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
intermediate formation, ingold: bimolecular nucleophilic addition
Step 15. The tetrahedral intermediate collapses, eliminating the Cys164 sidechain. The position of Phe215 postulates its involvement in driving the decomposition of the tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Phe215A | polar/non-polar interaction |
| Cys164A | activator |
| His303A | hydrogen bond donor |
| Asn336A | hydrogen bond donor, electrostatic stabiliser |
| Cys164A | nucleofuge |
Chemical Components
enzyme-substrate complex cleavage, intermediate formation, elimination (not covered by the Ingold mechanisms)
Step 16. Cys164 attacks at the thio-carbonyl, reforming an enzyme-substrate tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys164A | activator |
| His303A | hydrogen bond donor |
| Asn336A | hydrogen bond donor, electrostatic stabiliser |
| Cys164A | nucleophile |
Chemical Components
enzyme-substrate complex formation, intermediate formation, ingold: bimolecular nucleophilic addition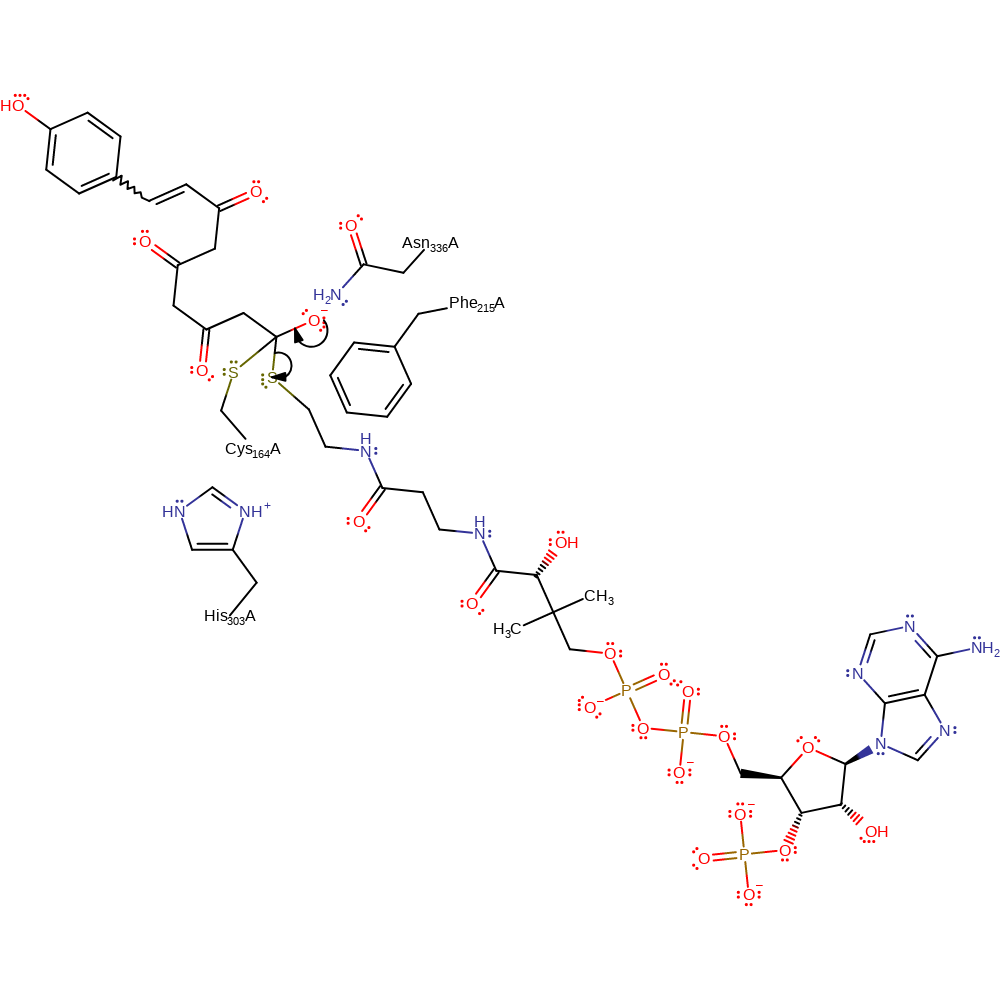
Step 17. The tetrahedral intermediate collapses, eliminating coenzyme A and forming the enzyme-bonded mono-ketide product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys164A | covalently attached, activator |
| His303A | hydrogen bond donor |
| Asn336A | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
overall product formed, intermediate formation, elimination (not covered by the Ingold mechanisms)
Step 18. An intramolecular proton-rearrangment occurs during cyclisation.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached |
Chemical Components
ingold: intramolecular nucleophilic addition, proton transfer, intermediate formation, cyclisation
Step 19. The Cys164 side chain is eliminated, resulting in the formation of a cyclic tri-ketide. In the absence of chalcone isomerase, the enzyme-bound polyketide undergoes non-stereospecific C-ring closure [DOI:10.1021/ja0027113]. The involvement of water in abstracting a proton, as shown here, is inferred.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | electrostatic stabiliser, hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator, covalently attached, nucleofuge |
Chemical Components
ingold: intramolecular nucleophilic addition, intermediate formation, inferred reaction step
Step 20. The triketide ring undergoes intramolecular proton transfer to give an aromatic ring.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Asn336A | hydrogen bond donor |
| His303A | hydrogen bond donor |
| Cys164A | activator |





 Download:
Download: