Strictosidine synthase
Strictosidine synthase is an unusual six-bladed beta-propeller fold protein which catalyses the condensation of two precursor molecules, tryptamine and monoterpenoid secologanin to form strictosidine. This molecule is the generic starting material for the biosynthetic formation of monoterpenoid-derived indole alkaloids, many of which are known to have potent therapeutic characteristics, including anti-cancer properties.
Reference Protein and Structure
- Sequence
-
P68175
 (4.3.3.2)
(4.3.3.2)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Rauvolfia serpentina (serpentwood)

- PDB
-
2fp8
- Structure of Strictosidine Synthase, the Biosynthetic Entry to the Monoterpenoid Indole Alkaloid Family
(2.3 Å)



- Catalytic CATH Domains
-
2.120.10.30
 (see all for 2fp8)
(see all for 2fp8)
Enzyme Reaction (EC:4.3.3.2)
Enzyme Mechanism
Introduction
The enzyme catalyses the biological equivalent of the Pictet-Spengler reaction, a reaction used to form synthetic equivalents of indole alkaloids of pharmaceutical interest.
Catalytic Residues Roles
| UniProt | PDB* (2fp8) | ||
| Glu309 | Glu309(287)A | Acts as a general acid/base. | hydrogen bond acceptor, hydrogen bond donor, proton acceptor, proton donor, activator, increase nucleophilicity |
Chemical Components
proton transfer, bimolecular nucleophilic addition, intermediate formation, overall reactant used, intramolecular elimination, schiff base formed, overall product formed, intramolecular nucleophilic addition, native state of enzyme regenerated, inferred reaction stepReferences
- Ma X et al. (2006), Plant Cell, 18, 907-920. The Structure of Rauvolfia serpentina Strictosidine Synthase Is a Novel Six-Bladed -Propeller Fold in Plant Proteins. DOI:10.1105/tpc.105.038018. PMID:16531499.
- Klausen RS et al. (2017), J Am Chem Soc, 139, 12299-12309. Chiral Thioureas Promote Enantioselective Pictet-Spengler Cyclization by Stabilizing Every Intermediate and Transition State in the Carboxylic Acid-Catalyzed Reaction. DOI:10.1021/jacs.7b06811. PMID:28787140.
- Stöckigt J et al. (2008), Plant Physiol Biochem, 46, 340-355. 3D-Structure and function of strictosidine synthase – the key enzyme of monoterpenoid indole alkaloid biosynthesis. DOI:10.1016/j.plaphy.2007.12.011. PMID:18280746.

Step 1. Glu309 deprotonates the primary amine of tryptamine, initiating nucleophilic attack at the aldehyde group of secologanin.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | increase nucleophilicity, hydrogen bond acceptor, proton acceptor |
Chemical Components
proton transfer, ingold: bimolecular nucleophilic addition, intermediate formation, overall reactant usedCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | hydrogen bond donor |
Chemical Components
proton transfer, intermediate formation
Step 3. The secondary amine eliminates water, forming a Schiff base intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | activator, hydrogen bond donor, proton donor |
Chemical Components
proton transfer, ingold: intramolecular elimination, schiff base formed, overall product formed, intermediate formation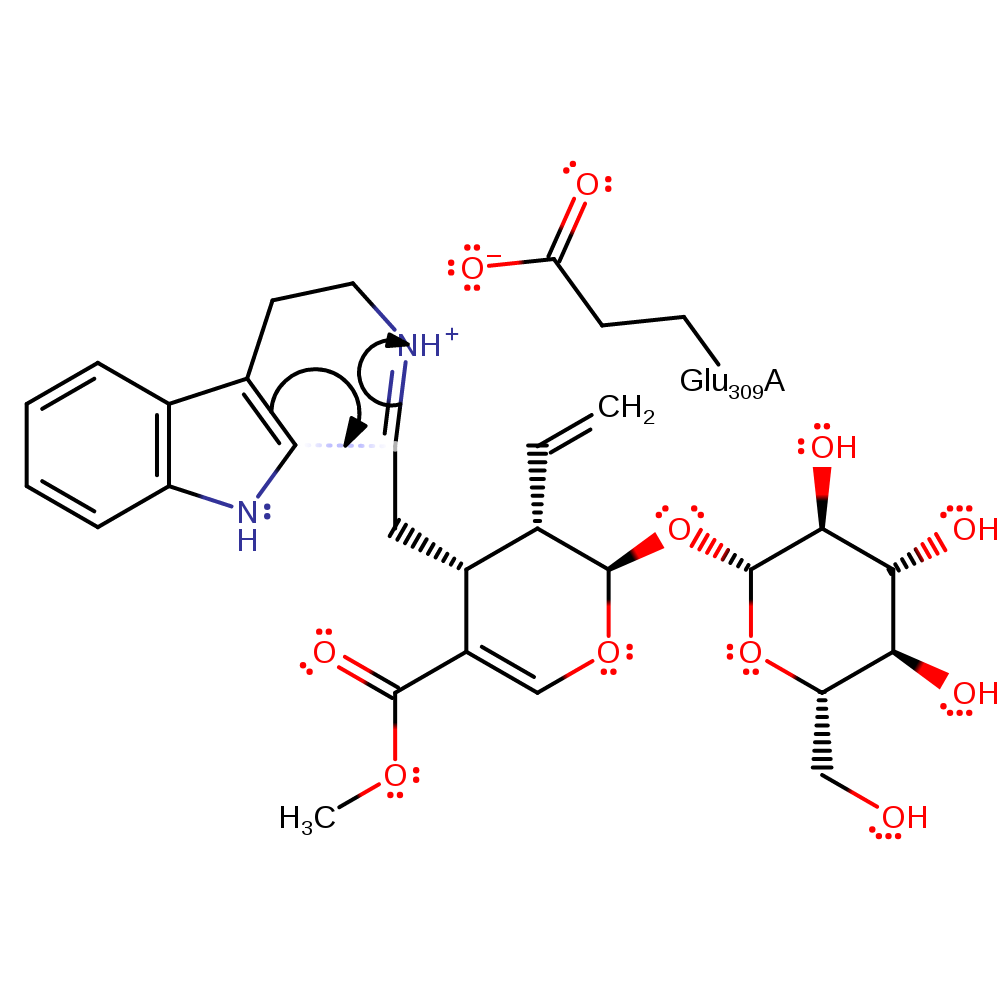
Step 4. The indole double bond attacks the imine, forming a carbon-carbon bond and a carbocation stabilised through conjugation.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | hydrogen bond acceptor |
Chemical Components
ingold: intramolecular nucleophilic addition, intermediate formation
Step 5. The Glu309 abstracts the proton alpha to the carbocation, forming a double bond.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | activator, hydrogen bond acceptor, proton acceptor |
Chemical Components
proton transfer, intermediate formation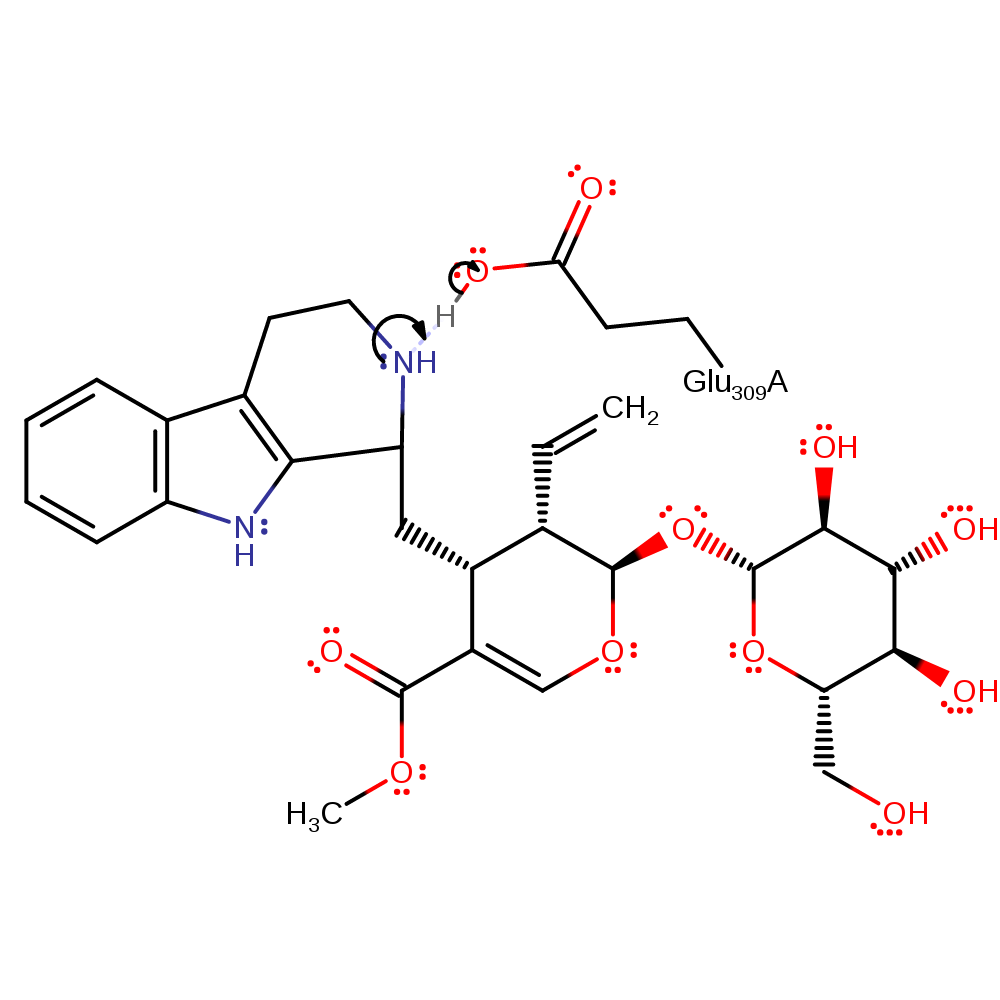
Step 6. The secondary amide deprotonates Glu309, regenerating the active site. The active site is burried, preventing access of solvent molecules to the intermediates formed during the reaction. Therefore, the product is shown here as deprotonating the general base, to allow for further catalysis, rather than proton abstraction by a solvent molecule [PMID:18280746].
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu309(287)A | activator, hydrogen bond donor, proton donor |





 Download:
Download: