Thiamine phosphate synthase
Thiamin phosphate synthase catalyses the formation of thiamin phosphate from 4-amino-5-(hydroxymethyl)-2-methylpyrimidine pyrophosphate and 5-(hydroxyethyl-4-methylthiazole phosphate, in the penultimate step of the biosynthesis of thiamin phosphate.
Reference Protein and Structure
- Sequence
-
P39594
 (2.5.1.3)
(2.5.1.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Bacillus subtilis subsp. subtilis str. 168 (Bacteria)

- PDB
-
2tps
- THIAMIN PHOSPHATE SYNTHASE
(1.25 Å)



- Catalytic CATH Domains
-
3.20.20.70
 (see all for 2tps)
(see all for 2tps)
- Cofactors
- Magnesium(2+) (1)
Enzyme Reaction (EC:2.5.1.3)
Enzyme Mechanism
Introduction
The reaction of thiamin phosphate synthase occurs by a dissociative SN1 mechanism. Ser 130 activates the pyrophosphate as a leaving group and transition state stabilisation is maintained by a magnesium cation. The ionised group is then able to react with 5-(hydroxyethyl-4-methylthiazole phosphate to give thiamin phosphate. The carbocation intermediate is stabilised by the counterion and a hydrogen bond between the pyrimidine amino group and the pyrophosphate.
Catalytic Residues Roles
| UniProt | PDB* (2tps) | ||
| Ser117 | Ser130(122)A | Activates the pyrophosphate to act as a leaving group. | enhance reactivity, electrostatic stabiliser |
| Arg46, Lys146 | Arg59(51)A, Lys159(151)A | Help stabilise the reactive intermediates formed during the course of the reaction. | electrostatic stabiliser |
Chemical Components
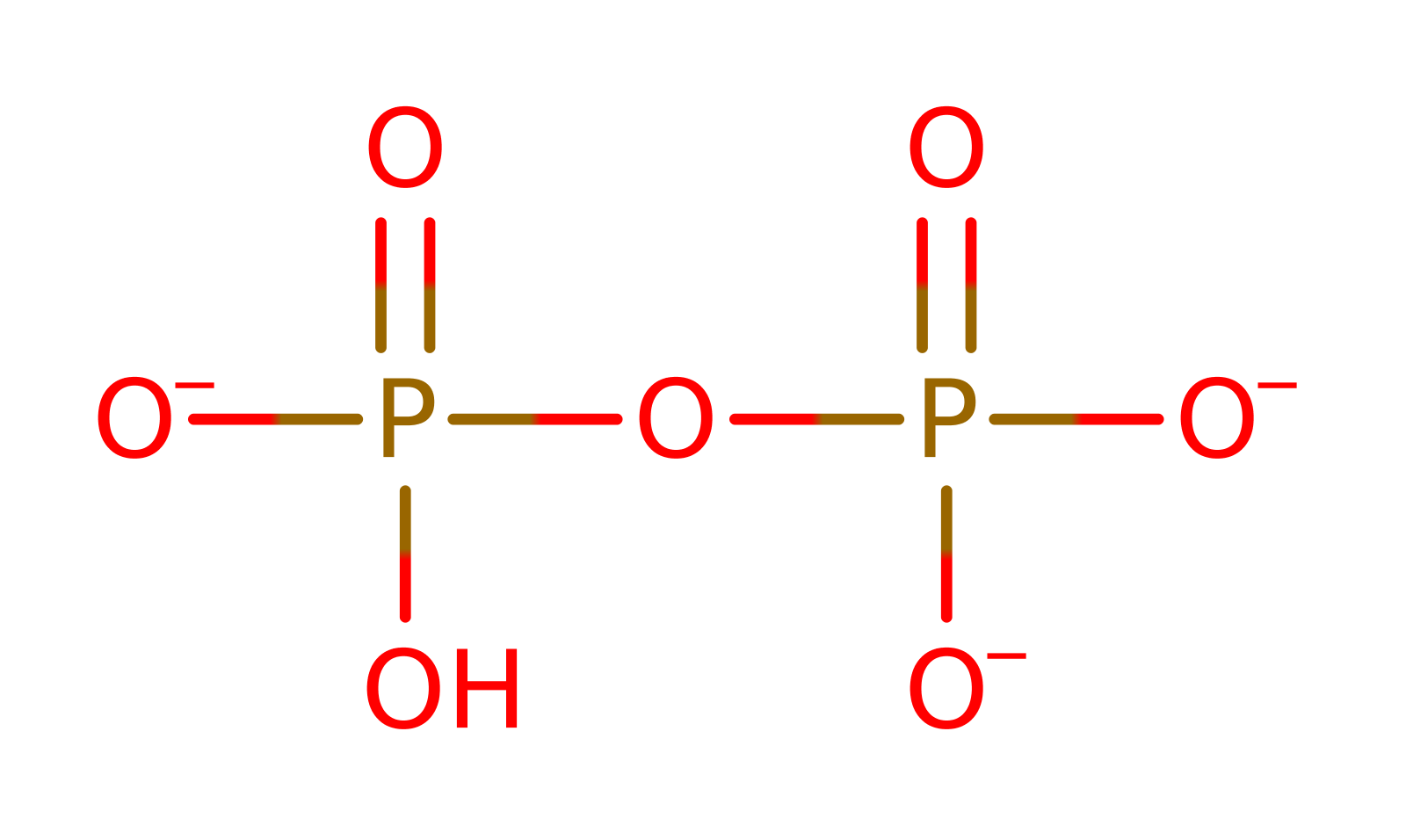
rate-determining step, unimolecular elimination by the conjugate base, overall reactant used, overall product formed, bimolecular nucleophilic additionReferences
- Peapus DH et al. (2001), Biochemistry, 40, 10103-10114. Structural Characterization of the Enzyme−Substrate, Enzyme−Intermediate, and Enzyme−Product Complexes of Thiamin Phosphate Synthase†,‡. DOI:10.1021/bi0104726. PMID:11513589.
- Jurgenson CT et al. (2009), Annu Rev Biochem, 78, 569-603. The Structural and Biochemical Foundations of Thiamin Biosynthesis. DOI:10.1146/annurev.biochem.78.072407.102340. PMID:19348578.
- Reddick JJ et al. (2001), Biochemistry, 40, 10095-10102. Mechanistic Studies on Thiamin Phosphate Synthase: Evidence for a Dissociative Mechanism†. DOI:10.1021/bi010267q. PMID:11513588.
- Chiu HJ et al. (1999), Biochemistry, 38, 6460-6470. Crystal Structure of Thiamin Phosphate Synthase fromBacillus subtilisat 1.25 Å Resolution†,‡. DOI:10.1021/bi982903z. PMID:10350464.
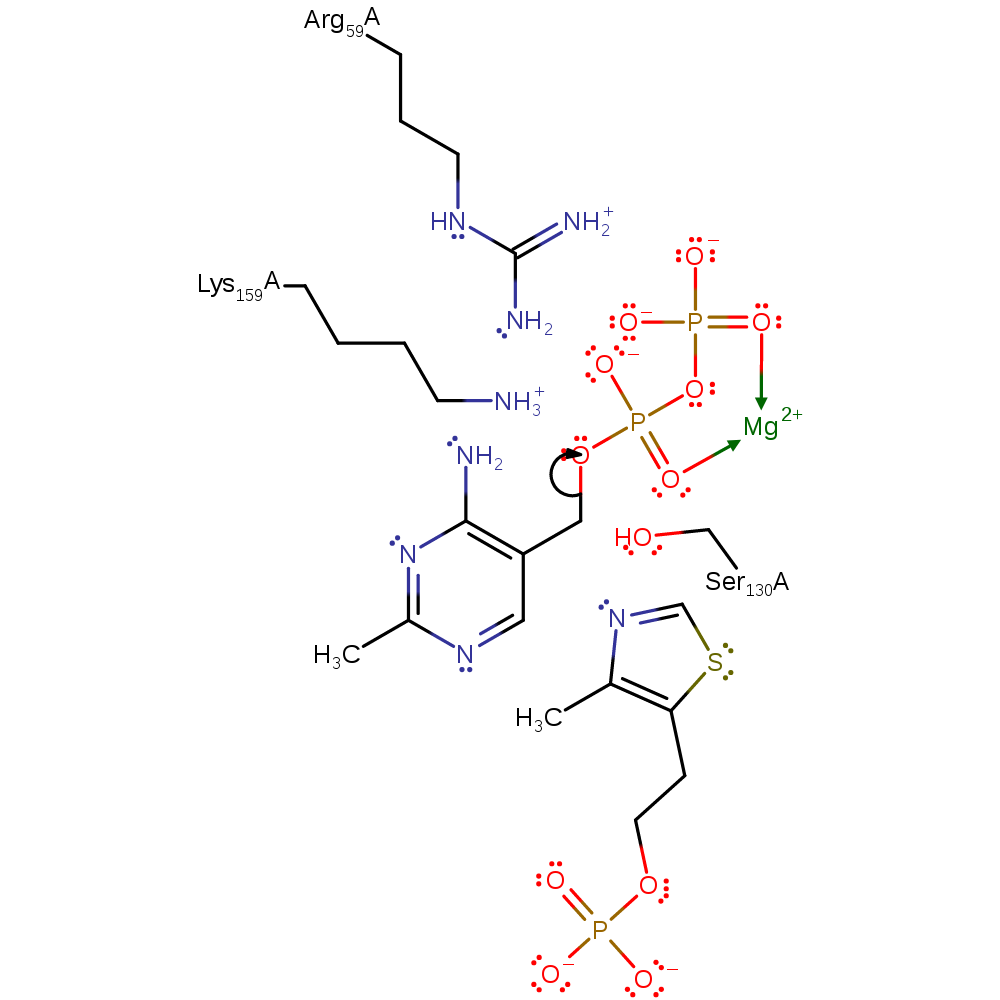
Step 1. Ser130 activates the pyrophosphate as a leaving group and transition state stabilisation is maintained by a magnesium cation.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg59(51)A | electrostatic stabiliser |
| Ser130(122)A | electrostatic stabiliser |
| Lys159(151)A | electrostatic stabiliser |
| Ser130(122)A | enhance reactivity |
Chemical Components
rate-determining step, ingold: unimolecular elimination by the conjugate base, overall reactant used, overall product formed
Step 2. The ionised carbocation is then able to react with 5-(hydroxyethyl-4-methylthiazole phosphate to give thiamin phosphate. The intermediate is stabilised by a hydrogen bond between the pyrimidine amino group and the pyrophosphate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg59(51)A | electrostatic stabiliser |
| Ser130(122)A | electrostatic stabiliser |
| Lys159(151)A | electrostatic stabiliser |




 Download:
Download: