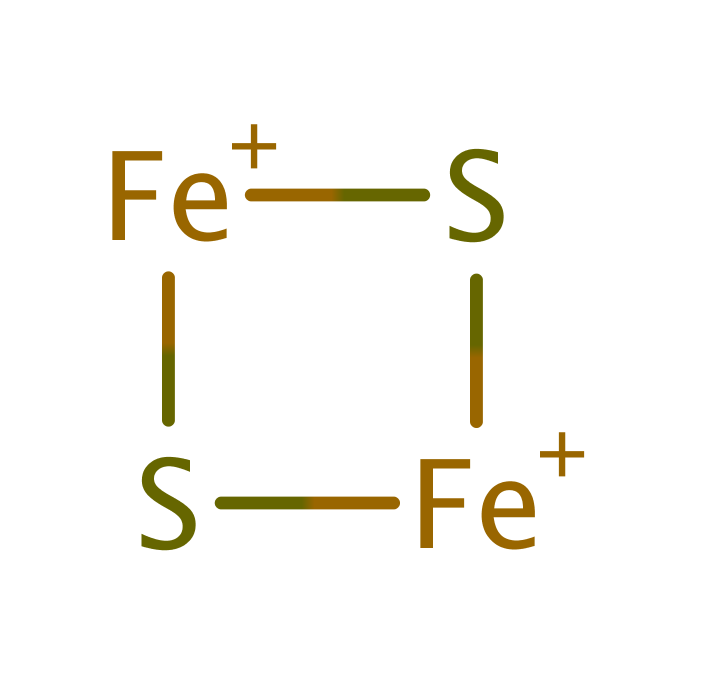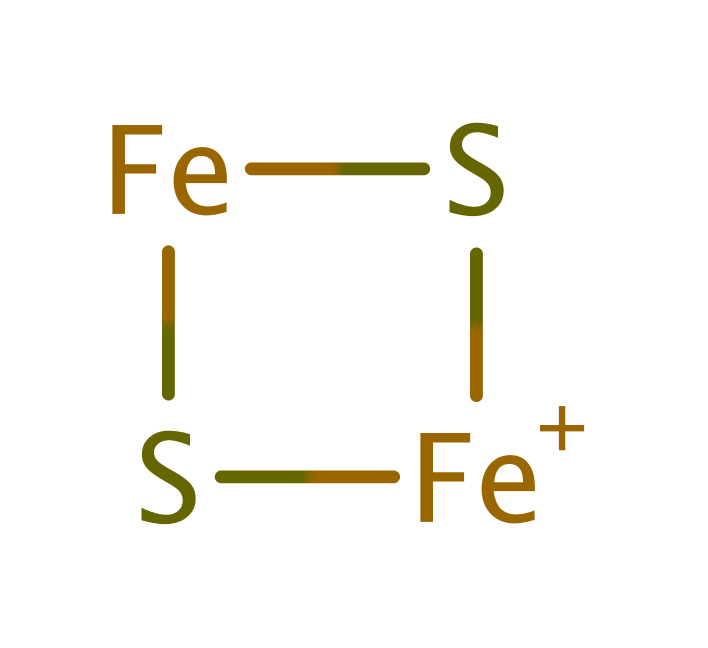Ferredoxin--NADP reductase
Ferredoxin reductase (FNR) catalyses the final electron transport step of linear photosynthesis, receiving one electron from two electron carrier ferredoxin proteins and uses these electrons to convert NADP+ into NADPH via hydride transfer from atom N(5) of the FAD cofactor using two molecules of ferredoxin (Fd) as the source of the electrons.
In chloroplasts and cyanobacteria the enzyme acts on plant-type [2Fe-2S] ferredoxins, but in other bacteria it can also reduce bacterial 2[4Fe-4S] ferredoxins and flavodoxin.
FNR is a member of a large family of electron transport proteins containing NAD and FAD binding sites which form an FNR-like module.
Reference Protein and Structure
- Sequence
-
P00455
 (1.18.1.2)
(1.18.1.2)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Spinacia oleracea (spinach)

- PDB
-
1fnb
- REFINED CRYSTAL STRUCTURE OF SPINACH FERREDOXIN REDUCTASE AT 1.7 ANGSTROMS RESOLUTION: OXIDIZED, REDUCED, AND 2'-PHOSPHO-5'-AMP BOUND STATES
(1.7 Å)



- Catalytic CATH Domains
-
2.40.30.10
 3.40.50.80
3.40.50.80  (see all for 1fnb)
(see all for 1fnb)
- Cofactors
- Fadh2(2-) (1)
Enzyme Reaction (EC:1.18.1.2)
Enzyme Mechanism
Introduction
The FAD cofactor is the functional catalytic group, however the residues around it are conserved and have roles in bonding, orientation, proton transfer and modification of the reduction potential at the FAD.
Ferredoxin (Fd) transfers an electron to FAD via the pi-system of Phe65 of Fd. A proton is transferred from the hydroxyl group of Fd S54, through Glu312 and Ser96, to the isoalloxazine N5 position to produce neutral semiquinone. A second molecule of Fd transfers an electron to the semiquinone to produce anionic hydroquinone. A hydride is transferred from the N5 position of the hydroquinone to the C4 position of NADP+ to produce FAD and NADPH.
Catalytic Residues Roles
| UniProt | PDB* (1fnb) | ||
| Tyr150 | Tyr95A | Helps modulate the midpoint potential of FAD. Along with Tyr314 binds the FAD via hydrophobic interactions between the benzene ring side chains and the isoalloxazine ring of FAD. | steric locator, alter redox potential |
| Ser151 | Ser96A | Conserved as a serine or a threonine amongst the superfamily. It acts to relay a proton from Glu312 to the N(5) position of FAD during the reduction by ferredoxin. It is also thought to stabilise the interaction between the isoalloxazine ring of FAD and the nicotinamide ring of NADP(H), so lowering the transition state energy associated with hydride transfer between the two molecules, and so facilitating the reduction reaction. | proton shuttle (general acid/base) |
| Cys327 | Cys272A | The residue is positioned within the active site to orientate the nicotinamide and isoalloxazine rings of NADP and FAD and aid hydride transfer between the molecules. | alter redox potential, electrostatic stabiliser |
| Glu367 | Glu312A | The residue is thought to relay a proton from the solvent to Ser96 to yield a neutral semi-quinone. The residue stabilises the semiquinone radical by forming a hydrogen bond to the isoalloxazine N5. It also destabilises the anionic hydroquinone and so acts to modify the FAD reduction potential. | proton shuttle (general acid/base), alter redox potential, electrostatic stabiliser |
| Tyr369 | Tyr314A | Although this C-terminal tyrosine is not thought to be directly involved in the hydride transfer, it is critical to the steady-state turnover of the enzyme. Along with Tyr95 binds the FAD via hydrophobic interactions between the benzene ring side chains and the isoalloxazine ring of FAD. | steric locator, alter redox potential |
Chemical Components
References
- Carrillo N et al. (2003), Eur J Biochem, 270, 1900-1915. Open questions in ferredoxin-NADP+ reductase catalytic mechanism. DOI:10.1046/j.1432-1033.2003.03566.x.
- Sánchez-Azqueta A et al. (2014), Biochim Biophys Acta, 1837, 296-305. External loops at the ferredoxin-NADP+ reductase protein–partner binding cavity contribute to substrates allocation. DOI:10.1016/j.bbabio.2013.11.016. PMID:24321506.
- Sánchez-Azqueta A et al. (2014), Biochim Biophys Acta, 1837, 251-263. A hydrogen bond network in the active site of Anabaena ferredoxin-NADP+ reductase modulates its catalytic efficiency. DOI:10.1016/j.bbabio.2013.10.010. PMID:24200908.
- Lans I et al. (2012), J Am Chem Soc, 134, 20544-20553. Theoretical Study of the Mechanism of the Hydride Transfer between Ferredoxin–NADP+Reductase and NADP+: The Role of Tyr303. DOI:10.1021/ja310331v. PMID:23181670.
- Lans I et al. (2010), J Phys Chem B, 114, 3368-3379. Mechanism of the Hydride Transfer betweenAnabaenaTyr303Ser FNRrd/FNRoxand NADP+/H. A Combined Pre-Steady-State Kinetic/Ensemble-Averaged Transition-State Theory with Multidimensional Tunneling Study. DOI:10.1021/jp912034m. PMID:20163096.
- Medina M (2009), FEBS J, 276, 3942-3958. Structural and mechanistic aspects of flavoproteins: photosynthetic electron transfer from photosystem I to NADP+. DOI:10.1111/j.1742-4658.2009.07122.x. PMID:19583765.
- Musumeci MA et al. (2008), FEBS J, 275, 1350-1366. Modulation of the enzymatic efficiency of ferredoxin-NADP(H) reductase by the amino acid volume around the catalytic site. DOI:10.1111/j.1742-4658.2008.06298.x. PMID:18279389.
- Tejero J et al. (2007), Arch Biochem Biophys, 459, 79-90. Catalytic mechanism of hydride transfer between NADP+/H and ferredoxin-NADP+ reductase from Anabaena PCC 7119. DOI:10.1016/j.abb.2006.10.023. PMID:17224127.
- Tejero J et al. (2005), Biochemistry, 44, 13477-13490. C-Terminal Tyrosine of Ferredoxin−NADP+Reductase in Hydride Transfer Processes with NAD(P)+/H†. DOI:10.1021/bi051278c. PMID:16216071.
- Faro M et al. (2002), Eur J Biochem, 269, 2656-2661. Role of critical charged residues in reduction potential modulation of ferredoxin-NADP+reductase. DOI:10.1046/j.1432-1033.2002.02925.x. PMID:12047373.
- Hermoso JA et al. (2002), J Mol Biol, 319, 1133-1142. Mechanism of Coenzyme Recognition and Binding Revealed by Crystal Structure Analysis of Ferredoxin–NADP+ Reductase Complexed with NADP+. DOI:10.1016/s0022-2836(02)00388-1. PMID:12079352.
- Mayoral T et al. (2000), Proteins, 38, 60-69. Structural basis of the catalytic role of Glu301 in Anabaena PCC 7119 ferredoxin-NADP+ reductase revealed by x-ray crystallography. DOI:10.1002/(sici)1097-0134(20000101)38:1<60::aid-prot7>3.3.co;2-2. PMID:10651039.
- Morales R et al. (2000), EMBO Rep, 1, 271-276. A redox-dependent interaction between two electron-transfer partners involved in photosynthesis. DOI:10.1093/embo-reports/kvd057. PMID:11256611.
- Aliverti A et al. (1998), J Biol Chem, 273, 34008-34015. Probing the function of the invariant glutamyl residue 312 in spinach ferredoxin-NADP+ reductase. PMID:9852055.
- Serre L et al. (1996), J Mol Biol, 263, 20-39. X-ray Structure of the Ferredoxin:NADP+Reductase from the CyanobacteriumAnabaenaPCC 7119 at 1.8 Å Resolution, and Crystallographic Studies of NADP+Binding at 2.25 Å Resolution. DOI:10.1006/jmbi.1996.0553. PMID:8890910.
- Aliverti A et al. (1995), Biochemistry, 34, 8371-8379. Involvement of Serine 96 in the Catalytic Mechanism of Ferredoxin-NADP+ Reductase: Structure-Function Relationship As Studied by Site-Directed Mutagenesis and X-ray Crystallography. DOI:10.1021/bi00026a019. PMID:7677850.
- Bruns CM et al. (1995), J Mol Biol, 247, 125-145. Refined crystal structure of spinach ferredoxin reductase at 1.7 A resolution: oxidized, reduced and 2'-phospho-5'-AMP bound states. DOI:10.2210/pdb1fnb/pdb. PMID:7897656.
- Aliverti A et al. (1993), Biochemistry, 32, 6374-6380. The role of cysteine residues of spinach ferredoxin-NADP+ reductase as assessed by site-directed mutagenesis. DOI:10.1021/bi00076a010. PMID:8518283.
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| Tyr95A | alter redox potential |
| Tyr314A | alter redox potential |
| Tyr95A | steric locator |
| Tyr314A | steric locator |
| Cys272A | alter redox potential |
| Glu312A | alter redox potential |
| Ser96A | proton shuttle (general acid/base) |
| Glu312A | proton shuttle (general acid/base), electrostatic stabiliser |
| Cys272A | electrostatic stabiliser |