Threonine---tRNA ligase
threonyl tRNA synthase from E.coli is able to catalyse the attachment of a threonine residue to its corresponding tRNA, displaying remarkable specificity as it only works on the specific amino acid and on the specific tRNA. It is part of the class II tRNA synthases, which make up approximately half of the known tRNA synthases. It is rare among class II synthases because of the presence of a Zinc ion in the active site, which is catalytic rather than structural.
Reference Protein and Structure
- Sequence
-
P0A8M3
 (6.1.1.3)
(6.1.1.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli K-12 (Bacteria)

- PDB
-
1qf6
- STRUCTURE OF E. COLI THREONYL-TRNA SYNTHETASE COMPLEXED WITH ITS COGNATE TRNA
(2.9 Å)



- Catalytic CATH Domains
-
3.30.930.10
 (see all for 1qf6)
(see all for 1qf6)
- Cofactors
- Zinc(2+) (1)
Enzyme Reaction (EC:6.1.1.3)
Enzyme Mechanism
Introduction
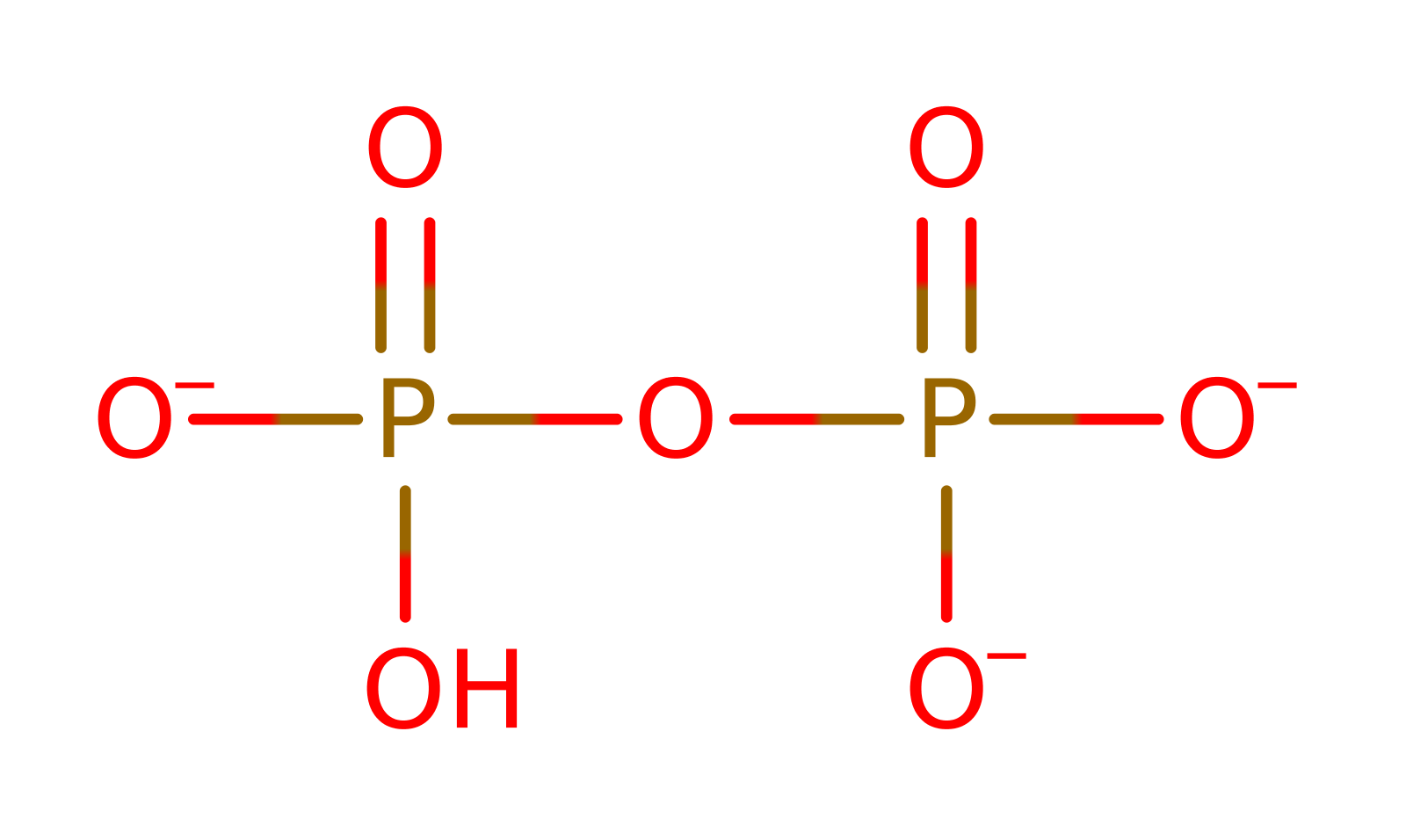
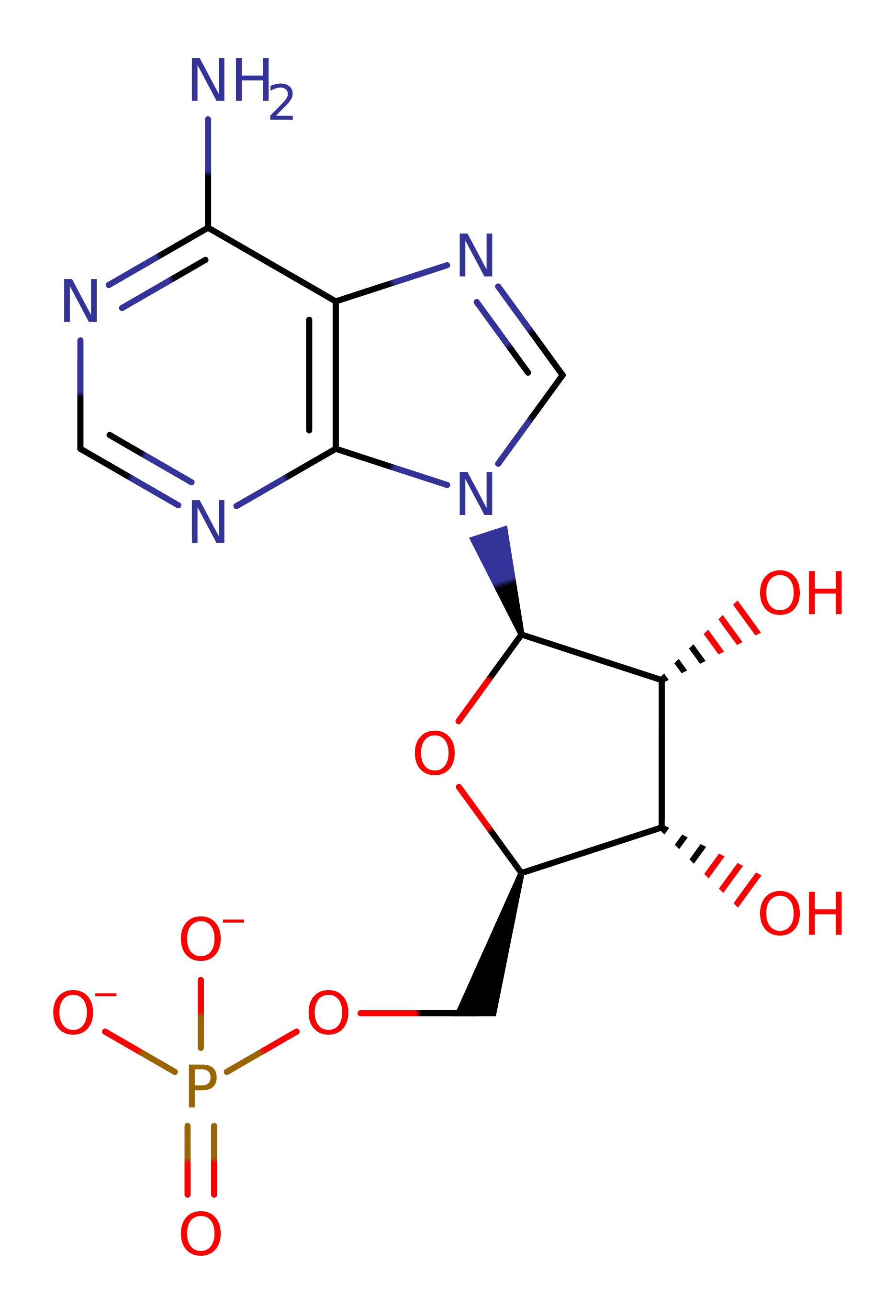
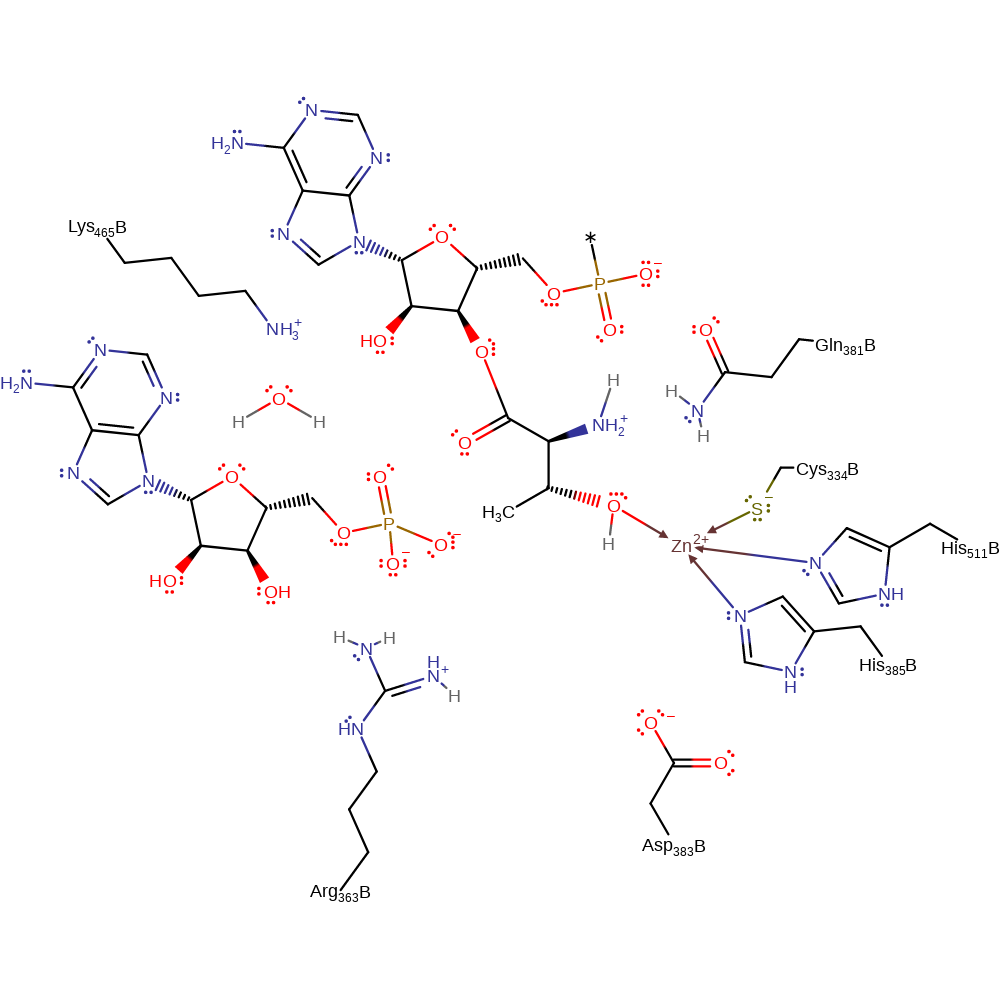
The reaction proceeds through direct nucleophilic attack from the carboxy terminal of the threonine on the alpha phosphate of ATP to form threonylAMP and releasing PPi in the process. This part of the reaction passes through a pentavalent phosphate intermediate, stabilised by Arg 363's positively charged side chain. The threonylAMP binds specifically to the Zinc ion, which therefore prevents other amino acids from forming amino acylAMPs. Placement of the tRNA anticodon allows specific transfer of the threonyl moiety to it by another nucleophilic addition elimination reaction, this time with AMP as the leaving group.
Catalytic Residues Roles
| UniProt | PDB* (1qf6) | ||
| Lys465, Gln381 | Lys465A(B), Gln381A(B) | The residues are thought to help stabilise negative charge during the reaction. | electrostatic stabiliser |
| Arg363 | Arg363A(B) | Positive charge on side chain interacts electrostatically with the pentavalent phosphate intermediate thus resulting in its stabilisation, facilitating the formation of the aminoacyl AMP. | electrostatic stabiliser |
| Cys334, His385, His511 | Cys334A(B), His385A(B), His511A(B) | Forms part of the catalytic zinc binding site. | metal ligand, electrostatic stabiliser |
Chemical Components
bimolecular nucleophilic substitution, intermediate formation, overall reactant used, proton transfer, intermediate collapse, overall product formedReferences
- Sankaranarayanan R et al. (1999), Cell, 97, 371-381. The Structure of Threonyl-tRNA Synthetase-tRNAThr Complex Enlightens Its Repressor Activity and Reveals an Essential Zinc Ion in the Active Site. DOI:10.1016/s0092-8674(00)80746-1. PMID:10319817.
- Aboelnga MM et al. (2017), J Phys Chem B, 121, 6163-6174. Roles of the Active Site Zn(II) and Residues in Substrate Discrimination by Threonyl-tRNA Synthetase: An MD and QM/MM Investigation. DOI:10.1021/acs.jpcb.7b03782. PMID:28592109.
- Perona JJ et al. (2014), Top Curr Chem, 344, 1-41. Synthetic and editing mechanisms of aminoacyl-tRNA synthetases. DOI:10.1007/128_2013_456. PMID:23852030.
- Torres-Larios A et al. (2003), J Mol Biol, 331, 201-211. Conformational movements and cooperativity upon amino acid, ATP and tRNA binding in threonyl-tRNA synthetase. PMID:12875846.
- Musier-Forsyth K et al. (2000), Nat Struct Biol, 7, 435-436. Role of zinc ion in translational accuracy becomes crystal clear. DOI:10.1038/75816. PMID:10881182.
- Sankaranarayanan R et al. (2000), Nat Struct Biol, 7, 461-465. Zinc ion mediated amino acid discrimination by threonyl-tRNA synthetase. DOI:10.1038/75856. PMID:10881191.

Step 1. The first step of the reaction involves nucleophilic attack from the carboxy terminal of the threonine on the alpha phosphate of the ATP. This forms threonylAMP and releases PPi. The reaction is stabilised by the positively charged Arg363 side chain.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys334A(B) | metal ligand |
| His385A(B) | metal ligand |
| His511A(B) | metal ligand |
| Arg363A(B) | electrostatic stabiliser |
| Gln381A(B) | electrostatic stabiliser |
| Asp383A(B) | electrostatic stabiliser |
| Lys465A(B) | electrostatic stabiliser |
Chemical Components
ingold: bimolecular nucleophilic substitution, intermediate formation, overall reactant used
Step 2. The amine group of threonylAMP deprotonates the 3'-OH group of the tRNA. This is followed by nucleophilic attack of the oxygen on the carbonyl group of the threoylAMP, eliminating AMP.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Cys334A(B) | electrostatic stabiliser |
| Arg363A(B) | electrostatic stabiliser |
| Gln381A(B) | electrostatic stabiliser |
| Asp383A(B) | electrostatic stabiliser |
| Cys334A(B) | metal ligand |
| His511A(B) | metal ligand |
| His385A(B) | metal ligand |





 Download:
Download: