Carnitine O-acetyltransferase
Carnitine acetyltransferase (CrAT) belongs to the family of choline/carnitine acyltransferases, which catalyse the exchange between acyl-CoA and acylcarnitine (likewise with choline). The enzymes of this family are classified based on their substrate selectivity. CrAT prefers short-chain fatty acids. CrAT maybe involved in the transport of acetyl-CoA across intercellular membranes, in maintaining the acetyl-CoA:CoA balance and in the excretion of excess or harmful acyl molecules as acylcarnitines. Inherited deficiency of CrAT activity can lead to serious neurological and heart problems, and patients suffering from Alzhiemer's disease also have reduced CrAT activity.
Reference Protein and Structure
- Sequence
-
P47934
 (2.3.1.7, 2.3.1.137)
(2.3.1.7, 2.3.1.137)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Mus musculus (house mouse)

- PDB
-
1ndi
- Carnitine Acetyltransferase in complex with CoA
(2.3 Å)



- Catalytic CATH Domains
-
3.30.559.40
 3.30.559.70
3.30.559.70  (see all for 1ndi)
(see all for 1ndi)
Enzyme Reaction (EC:2.3.1.7)
Enzyme Mechanism
Introduction
His343 acts as a general base to deprotonate the 3-hydroxyl group of the carnitine or the thiol group of CoA, depending on what is being acetylated. The activated hydroxyl or thiol group acts as a nucleophile to attack the carbonyl carbon in acyl-CoA or acylcarnitine. The oxyanion intermediate (with a negatively charged oxygen atom) is stabilised by Ser554, apart of the STS motif, which forms the oxyanion hole. The reaction mechanism also has the carnitine trimethylammonium group lowering the pKa around the attacking hydroxyl and also stabilising the oxyanion intermediate, an example of substrate-assisted catalysis. The same mechanism occurs with Choline O-Acetyltransferase
Catalytic Residues Roles
| UniProt | PDB* (1ndi) | ||
| Tyr107, Pro120 | Tyr107(78)A, Pro120(91)A | By analogy with choline O-acetyltransferase Tyr107 and Pro120 steric pack with His 334, which forces the His 334 to adopt a strained conformation with an intraresidue hydrogen bond between N-delta and its carbonyl oxygen. This is proposed to position N-epsilon and stabilise its non-protonated, nucleophilic state. This promotes it role as a general base in the first step of the reaction. | steric role |
| His343 | His343(314)A | It acts as a base to deprotonate the hydroxyl group of carnitine or the thiol group of CoA to promote their nucleophilic attack on the carbonyl carbon of CoA or carnitine. | hydrogen bond acceptor, proton acceptor, proton donor |
| Ser554 | Ser554(525)A | It stabilises the negatively charged intermediate by forming an oxyanion hole. | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
proton transfer, overall reactant used, intermediate formation, bimolecular nucleophilic addition, unimolecular elimination by the conjugate base, intermediate terminated, overall product formed, native state of enzyme regeneratedReferences
- Jogl G et al. (2003), Cell, 112, 113-122. Crystal Structure of Carnitine Acetyltransferase and Implications for the Catalytic Mechanism and Fatty Acid Transport. DOI:10.1016/s0092-8674(02)01228-x. PMID:12526798.
- Hsiao YS et al. (2006), J Biol Chem, 281, 28480-28487. Crystal structures of murine carnitine acetyltransferase in ternary complexes with its substrates. DOI:10.1074/jbc.M602622200. PMID:16870616.
- Kim AR et al. (2006), Biochemistry, 45, 14621-14631. Substrate binding and catalytic mechanism of human choline acetyltransferase. DOI:10.1021/bi061536l. PMID:17144655.
- Jogl G et al. (2004), Ann N Y Acad Sci, 1033, 17-29. Structure and function of carnitine acyltransferases. DOI:10.1196/annals.1320.002. PMID:15591000.
- Cai Y et al. (2004), EMBO J, 23, 2047-2058. Choline acetyltransferase structure reveals distribution of mutations that cause motor disorders. DOI:10.1038/sj.emboj.7600221. PMID:15131697.
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| His343(314)A | hydrogen bond acceptor |
| Tyr107(78)A | steric role |
| Pro120(91)A | steric role |
| His343(314)A | proton acceptor |
Chemical Components
proton transfer, overall reactant used
Step 2. Activated 3'OH then performs a nucleophilic attack on acetyl-CoA to form a tetrahedral intermediate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Ser554(525)A | electrostatic stabiliser |
| Ser554(525)A | hydrogen bond donor |
Chemical Components
intermediate formation, overall reactant used, ingold: bimolecular nucleophilic additionCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Ser554(525)A | electrostatic stabiliser, hydrogen bond donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, intermediate terminated, overall product formedCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His343(314)A | proton donor |



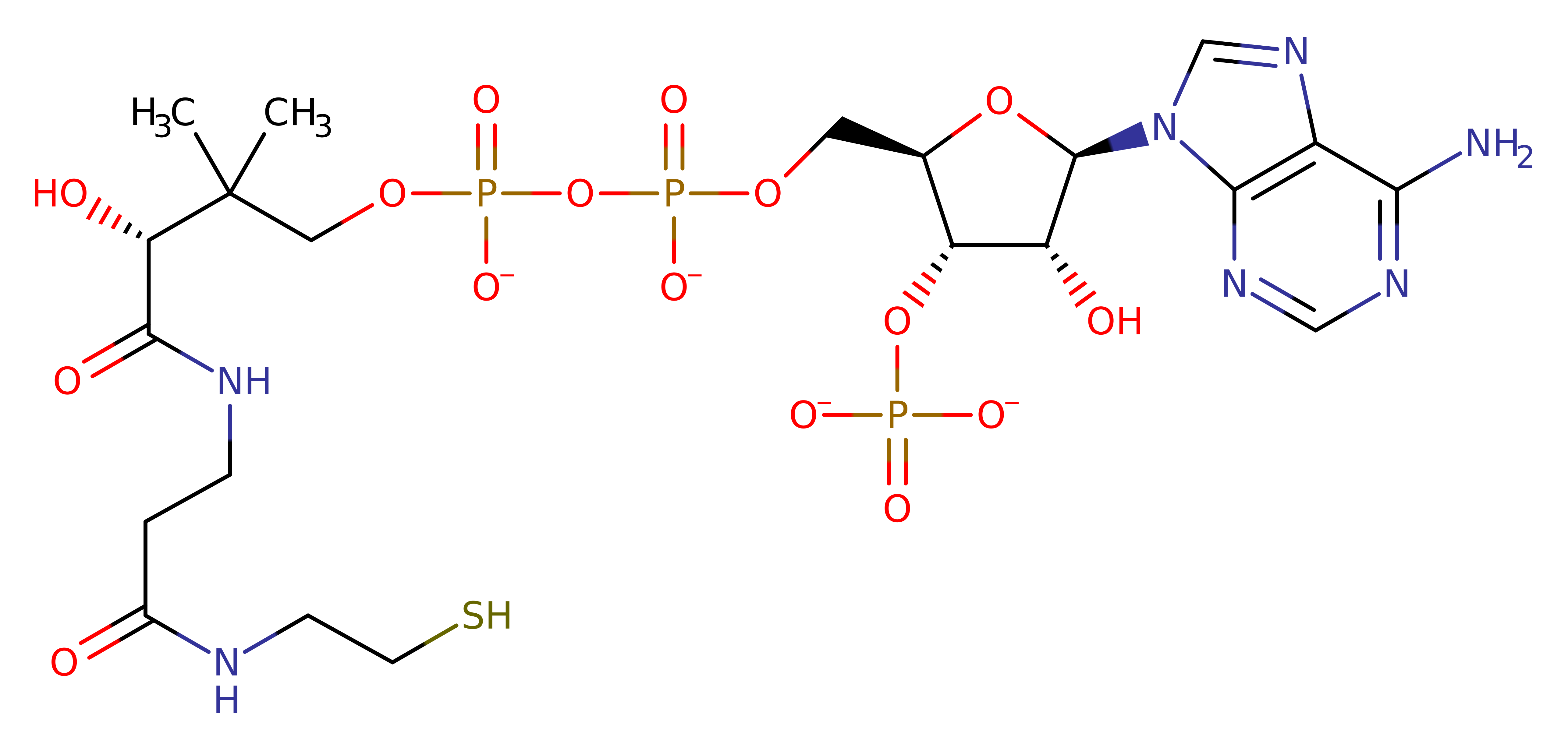



 Download:
Download: