Carbamate kinase
Carbamoyl phosphate (CP) can be synthesised from mixtures of ATP, bicarbonate and ammonia by two types of enzymes: carbamoyl phosphate synthases (CPS) and carbamate kinases (CK). CPS uses a three step mechanism and consumes two molecule of ATP per molecule of CP synthesised; this reaction is essentially irreversible and is the first committed step in the biosynthesis of pyrimidines, arginine and urea. By contrast, carbamate kinases (CK) make CP reversibly in a one-step reaction using one molecule of ATP per CP molecule synthesised. The actual substrate for these enzymes is carbamate which is in equilibrium with bicarbonate and ammonia. The in vivo role of CK from many organisms in which it has been studied (such as Enterococcus faecalis) seems to be to generate ATP from ADP using carbamoyl phosphate derived from the catabolism of arginine. However in some species, such as the hypothermophilic archaea Pyrococcus furiosus and Pyrococcus abyssi the enzyme appears to have an anabolic role. The enzyme from these species has in fact been called a "carbamate kinase-like carbamoyl phosphate synthetase", although studies have shown that is structurally and enzymologically a carbamate kinase.
Reference Protein and Structure
- Sequence
-
P0A2X8
 (2.7.2.2)
(2.7.2.2)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Enterococcus faecium (Bacteria)

- PDB
-
1b7b
- Carbamate kinase from Enterococcus faecalis
(2.8 Å)



- Catalytic CATH Domains
-
3.40.1160.10
 (see all for 1b7b)
(see all for 1b7b)
- Cofactors
- Magnesium(2+) (1)
Enzyme Reaction (EC:2.7.2.2)
Enzyme Mechanism
Introduction
Phosphoryl transfer from ATP to carbamate or from CP to ADP is proposed to occur via an in-line displacement reaction. The drawn out mechanism shows the formation of carbamate and ATP but the reverse reaction has the carbonyl on carbamate attacking the electrophilic phosphorous on gamma-phosphate of ATP, with the same catalytic residues and magnesium ion stabilising the transition state. More work is needed to further define the exact roles of the catalytic residues in order to be fully confident in the catalytic mechanism.
Catalytic Residues Roles
| UniProt | PDB* (1b7b) | ||
| Asn12, Gly11 (main-N) | Asn12A, Gly11A (main-N) | Proposed to neutralise negative charge on the phosphate on the carbamate during the reaction. | electrostatic stabiliser, polar interaction |
| Lys271 | Lys271A | Proposed to interact with the terminal phosphate of ADP and neutralise the developing negative charge on this group during the reaction. | electrostatic stabiliser, polar interaction |
| Lys128 | Lys128A | In the direction of CP breakdown, is proposed to interact with the bridging oxygen atom of CP and neutralise the developing negative charge on this oxygen atom as the product carbamate is generated. | electrostatic stabiliser, polar interaction |
| Lys209 | Lys209A | Proposed to stabilise negative charge in the transition state. | electrostatic stabiliser, polar interaction |
Chemical Components
bimolecular nucleophilic substitution, overall product formed, overall reactant usedReferences
- Ramón-Maiques S et al. (2010), J Mol Biol, 397, 1261-1275. Substrate binding and catalysis in carbamate kinase ascertained by crystallographic and site-directed mutagenesis studies: movements and significance of a unique globular subdomain of this key enzyme for fermentative ATP production in bacteria. DOI:10.1016/j.jmb.2010.02.038. PMID:20188742.
- Lim K et al. (2013), PLoS One, 8, e64004-. Crystal structures of carbamate kinase from Giardia lamblia bound with citric acid and AMP-PNP. DOI:10.1371/journal.pone.0064004. PMID:23700444.
- Ramón-Maiques S et al. (2002), Biochemistry, 41, 3916-3924. Molecular Physiology of Phosphoryl Group Transfer from Carbamoyl Phosphate by a Hyperthermophilic Enzyme at Low Temperature†. DOI:10.1021/bi011637d. PMID:11900534.
- Ramón-Maiques S et al. (2000), J Mol Biol, 299, 463-476. The 1.5 Å resolution crystal structure of the carbamate kinase-like carbamoyl phosphate synthetase from the hyperthermophilic archaeon Pyrococcus furiosus, bound to ADP, confirms that this thermostable enzyme is a carbamate kinase, and provides insight into substrate binding and stability in carbamate kinases. DOI:10.1006/jmbi.2000.3779. PMID:10860751.
- Uriarte M et al. (1999), J Biol Chem, 274, 16295-16303. The Carbamoyl-phosphate Synthetase of Pyrococcus furiosus Is Enzymologically and Structurally a Carbamate Kinase. DOI:10.1074/jbc.274.23.16295. PMID:10347186.
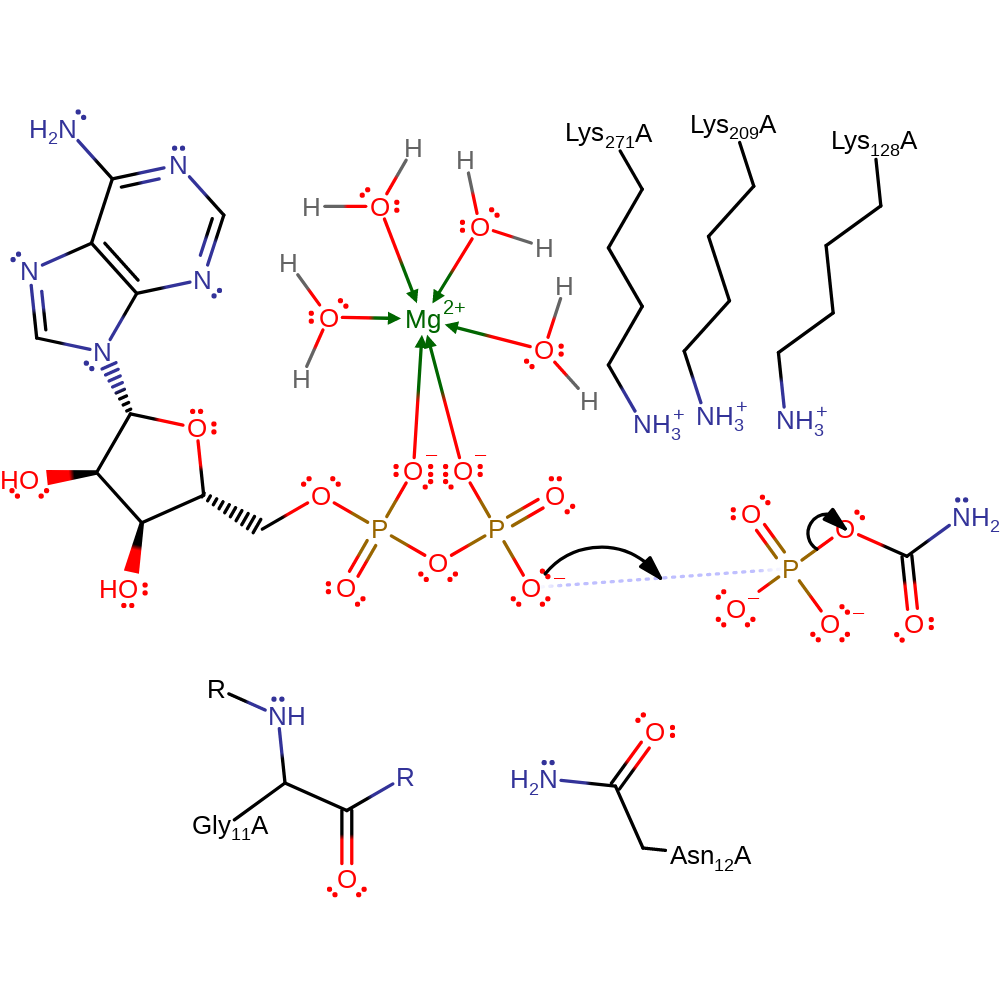
Step 1. The non-bridging oxygen on the ADP molecule beta-phosphate nucleophilically attacks the phosphorus on carbamoyl. The transition state observed in this nucleophilic substitution is stabilised by the surrounding residues Lys271, Lys209, Lys128, Gly11 and Asn12 and also Mg2+.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Lys209A | electrostatic stabiliser |
| Lys271A | electrostatic stabiliser |
| Lys128A | electrostatic stabiliser |
| Asn12A | electrostatic stabiliser |
| Gly11A (main-N) | electrostatic stabiliser |
| Gly11A (main-N) | polar interaction |
| Asn12A | polar interaction |
| Lys128A | polar interaction |
| Lys209A | polar interaction |
| Lys271A | polar interaction |
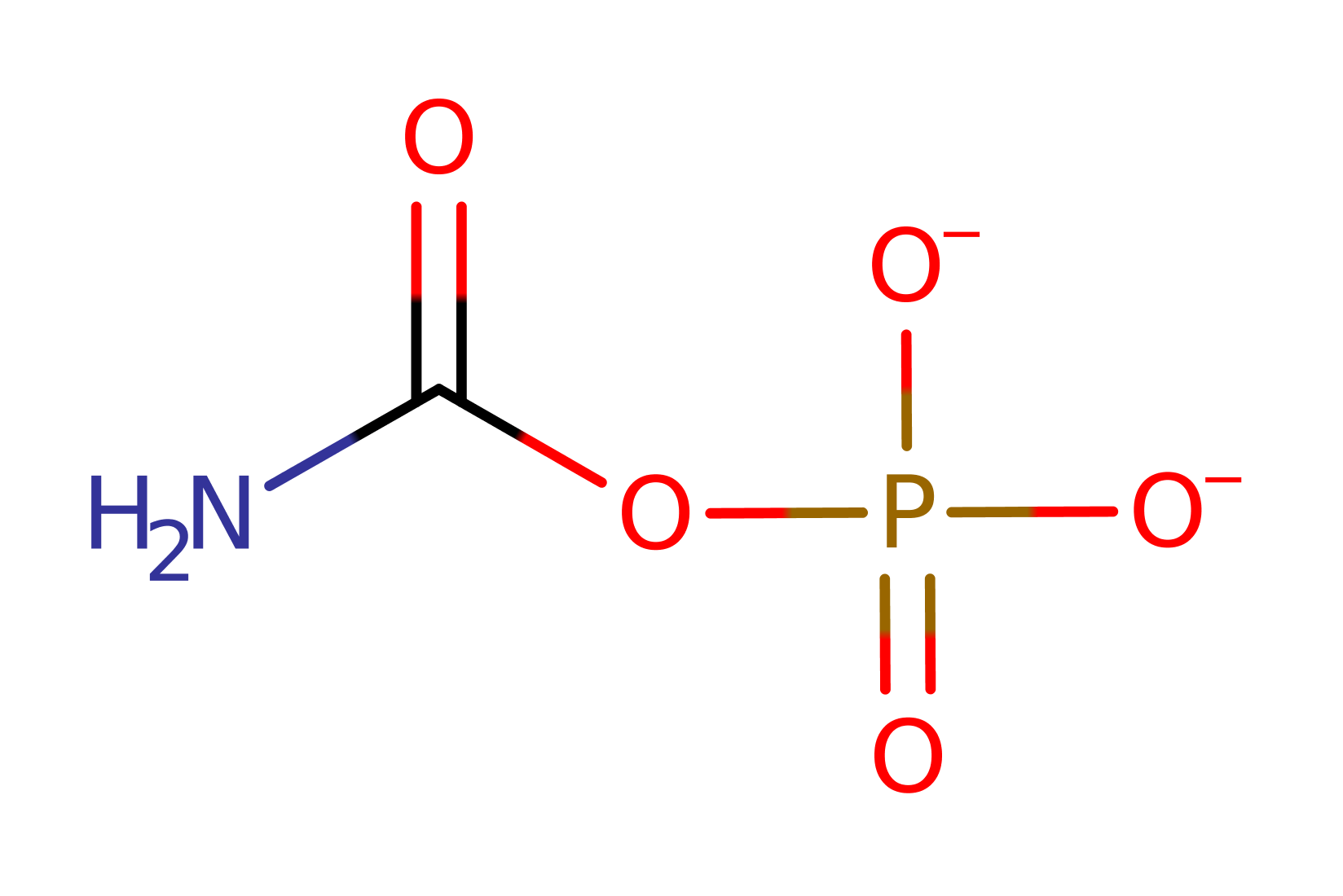


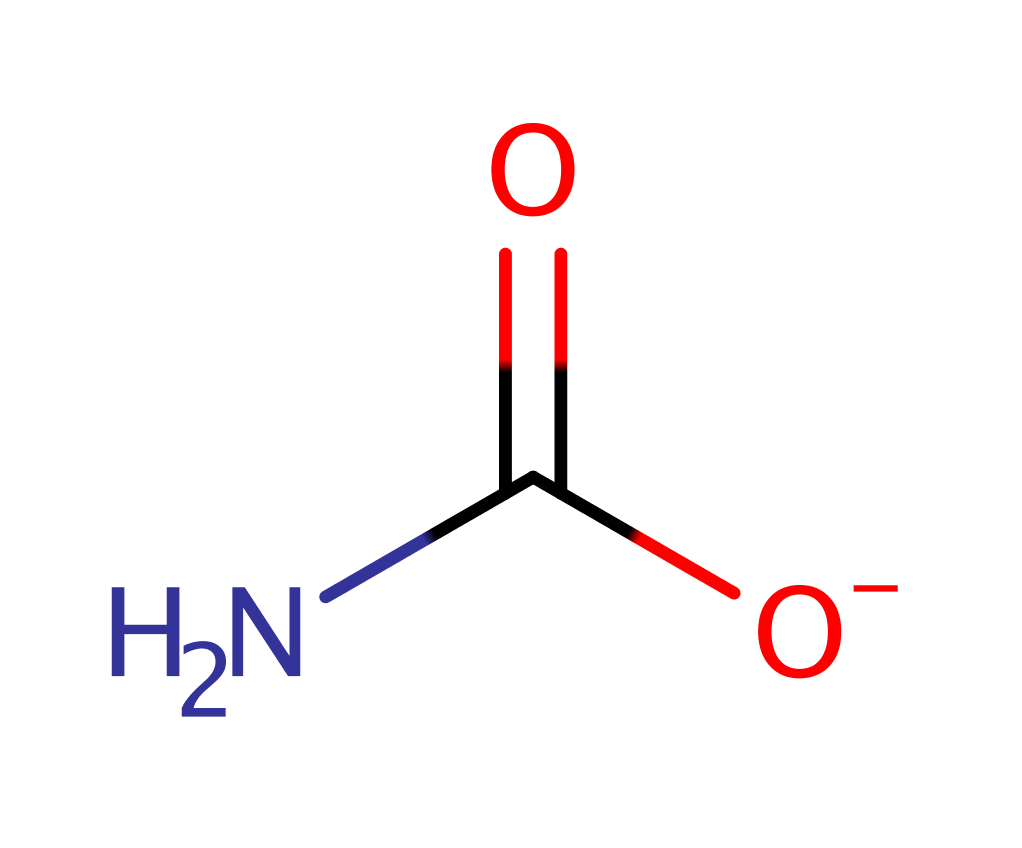
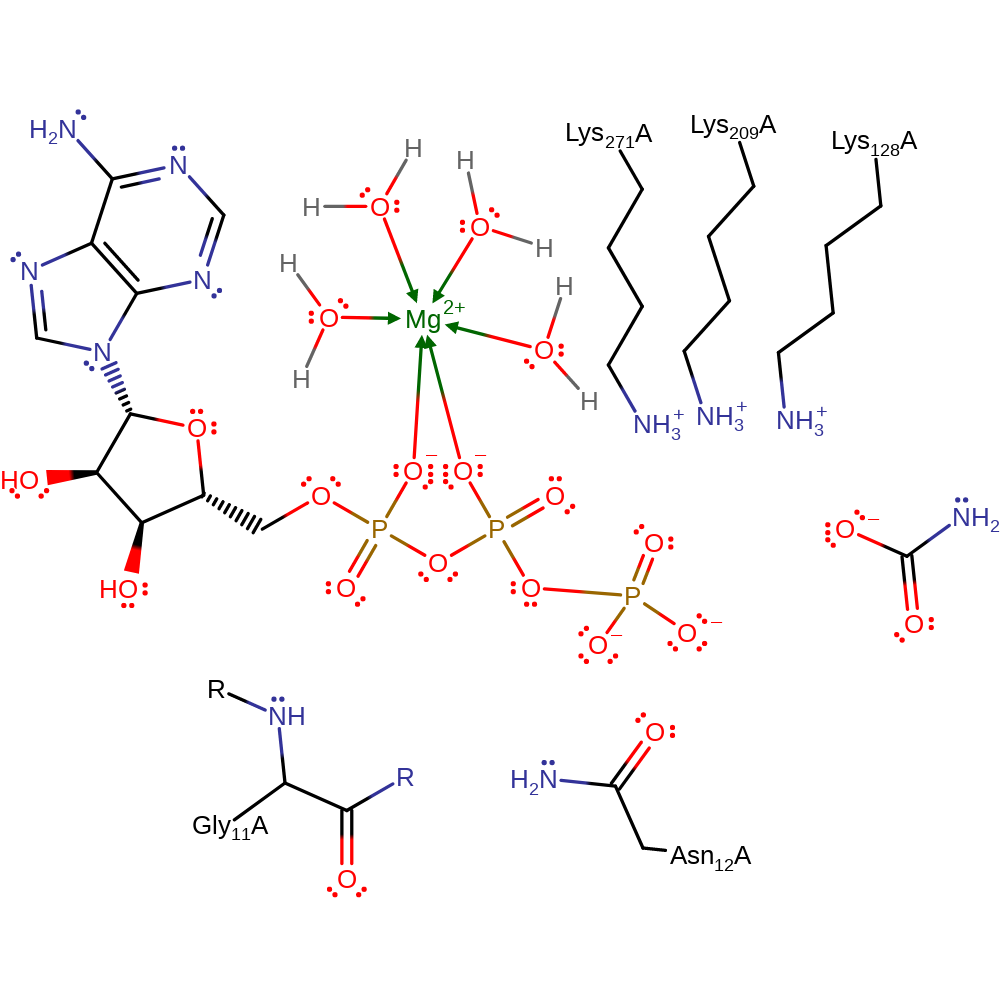 Download:
Download: