L-aspartate oxidase
L-aspartate oxidase (LASPO) is a flavo enzyme from Escherichia coli. It is unusual as it can use both oxygen and fumarate as electron acceptors for FAD oxidation, which means it is both aerobic and anaerobic. LASPO is an FAD dependent enzyme which catalyses the oxidation of L-aspartate into iminoaspartate. This is the first step in the bacterial synthesis of NAD+, and is a useful target for drug design, as this pathway is not present in mammals.
Reference Protein and Structure
- Sequence
-
P10902
 (1.4.3.16)
(1.4.3.16)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli K-12 (Bacteria)

- PDB
-
1knp
- E. coli L-aspartate oxidase: mutant R386L in complex with succinate
(2.6 Å)



- Catalytic CATH Domains
-
3.50.50.60
 3.90.700.10
3.90.700.10  (see all for 1knp)
(see all for 1knp)
- Cofactors
- Fadh2(2-) (1)
Enzyme Mechanism
Introduction
Arg 290 acts as a base by deprotonating C3 of L-aspartate. Arg 386 and His 351 stabilise the negative charge on the O4 oxygen atoms. FAD then accepts a hydride from C2 of L-aspartate (and most likely then accepts a proton from the solvent to form FADH2.)
If fumarate is used as the electron acceptor FADH- donates a hydride to the C2 atom of fumarate. The negative charge is localised on the O4 atoms which are stabilised by Arg386 and His 351. The C4 carbonyl is reformed, and Arg 290 donates a proton to the C3 atom, forming succinate.
Alternative molecular oxygen may re-oxidize FADH2, forming hydrogen peroxide.
Catalytic Residues Roles
| UniProt | PDB* (1knp) | ||
| Arg290 | Arg290A | Acts as a base and deprotonates the C3 atom of L-aspartate. Acts as an acid in its protonated form, and donates a proton to the C3 atom of fumerate. | proton acceptor, proton donor |
| Glu121, His244, Arg386, His351 | Glu121A, His244A, Leu386A, His351A | Acts to stabilise the negatively charged intermediate. | electrostatic stabiliser, steric role |
Chemical Components
proton transfer, hydride transfer, aromatic bimolecular nucleophilic addition, overall reactant used, cofactor used, bimolecular nucleophilic addition, native state of cofactor regeneratedReferences
- Bossi RT et al. (2002), Biochemistry, 41, 3018-3024. Structure of FAD-Boundl-Aspartate Oxidase: Insight into Substrate Specificity and Catalysis†,‡. DOI:10.1021/bi015939r. PMID:11863440.
- Tedeschi G et al. (2010), Biochimie, 92, 1335-1342. On the catalytic role of the active site residue E121 of E. coli L-aspartate oxidase. DOI:10.1016/j.biochi.2010.06.015. PMID:20600565.
- Messner KR et al. (2002), J Biol Chem, 277, 42563-42571. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. DOI:10.1074/jbc.M204958200. PMID:12200425.
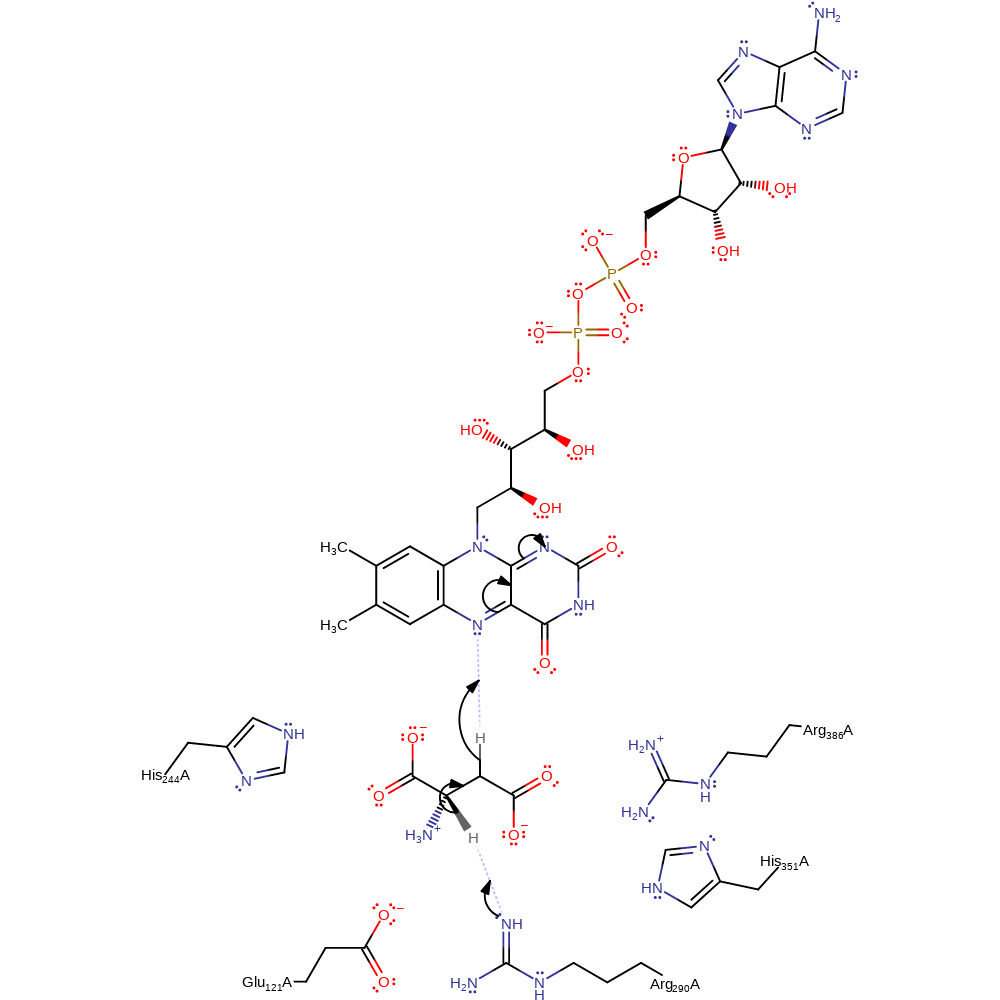
Step 1. Arg290 acts as a base to deprotonate C3 of the substrate. Glu121 orientates the substrate in a productive binding mode and promotes proton abstraction. FAD then accepts a hydride from C2, resulting in the enamine tautomer of the product and FADH-. The enamine will consequently tautomerise to form the imine product.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His351A | electrostatic stabiliser |
| Leu386A | electrostatic stabiliser |
| Glu121A | electrostatic stabiliser |
| His244A | electrostatic stabiliser |
| Glu121A | steric role |
| Arg290A | proton acceptor |
Chemical Components
proton transfer, hydride transfer, ingold: aromatic bimolecular nucleophilic addition, overall reactant used, cofactor used
Step 2. Fumarate reduction involved hydride transfer from FADH- N5 to fumarate C2, coupled with proton donation by Arg290. FAD and the active site residues are regenerated. Alternatively, molecular oxygen may be used to oxidise FADH- instead of fumarate (not shown here).
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg290A | proton donor |
Chemical Components
hydride transfer, proton transfer, ingold: bimolecular nucleophilic addition, native state of cofactor regeneratedIntroduction
Kinetic isotope effect experimental data argue against the succinate dehydrogenase-like mechanism for aspartate oxidation and for a concerted mechanism. The mechanism proposal shown here involves Glu121 may act as a base to deprotonate the amino group of L-aspartate, followed by hydride transfer from C2 to N5 of FAD. This forms the product and FADH-. It is also possible for the mechanism to involve deprotonation of C2 of L-aspartate followed by hydride transfer from N1 to FAD. FADH- may be reoxidised using fumarate, or may abstract a proton from the solvent to form FADH2 and be reoxidised by molecular oxygen.
Catalytic Residues Roles
| UniProt | PDB* (1knp) | ||
| Glu121 | Glu121A | Glu121 may act as a base to deprotonate L-aspartate in the first step of the mechanism. | proton acceptor, electrostatic stabiliser |
| Arg290 | Arg290A | Arg290 may act as a base to deprotonate L-aspartate in the first step of the mechanism. Arg290 is involved in the fumarate reduction reaction in which it acts as a general acid. | electrostatic stabiliser, proton donor |
| His244, Arg386, His351 | His244A, Leu386A, His351A | The residues act as electrostatic stabilisers. | electrostatic stabiliser |
Chemical Components
hydride transfer, proton transfer, aromatic bimolecular nucleophilic addition, overall reactant used, overall product formed, cofactor used, bimolecular nucleophilic addition, native state of cofactor regenerated, native state of enzyme regeneratedReferences
- Chow C et al. (2017), Biochemistry, 56, 4044-4052. Mechanistic Characterization of Escherichia coli l-Aspartate Oxidase from Kinetic Isotope Effects. DOI:10.1021/acs.biochem.7b00307. PMID:28700220.
- Bossi RT et al. (2002), Biochemistry, 41, 3018-3024. Structure of FAD-Boundl-Aspartate Oxidase: Insight into Substrate Specificity and Catalysis†,‡. DOI:10.1021/bi015939r. PMID:11863440.

Step 1. Glu121 binds to the protonated α-amino group of L-aspartate and acts as a base to perform the initial deprotonation of the amine. In a concerted reaction, the C2 hydride of L-aspartate is transferred to N5 of FAD. This results in the product and FADH-.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| His244A | electrostatic stabiliser |
| Arg290A | electrostatic stabiliser |
| His351A | electrostatic stabiliser |
| Glu121A | proton acceptor |
Chemical Components
hydride transfer, proton transfer, ingold: aromatic bimolecular nucleophilic addition, overall reactant used, overall product formed, cofactor used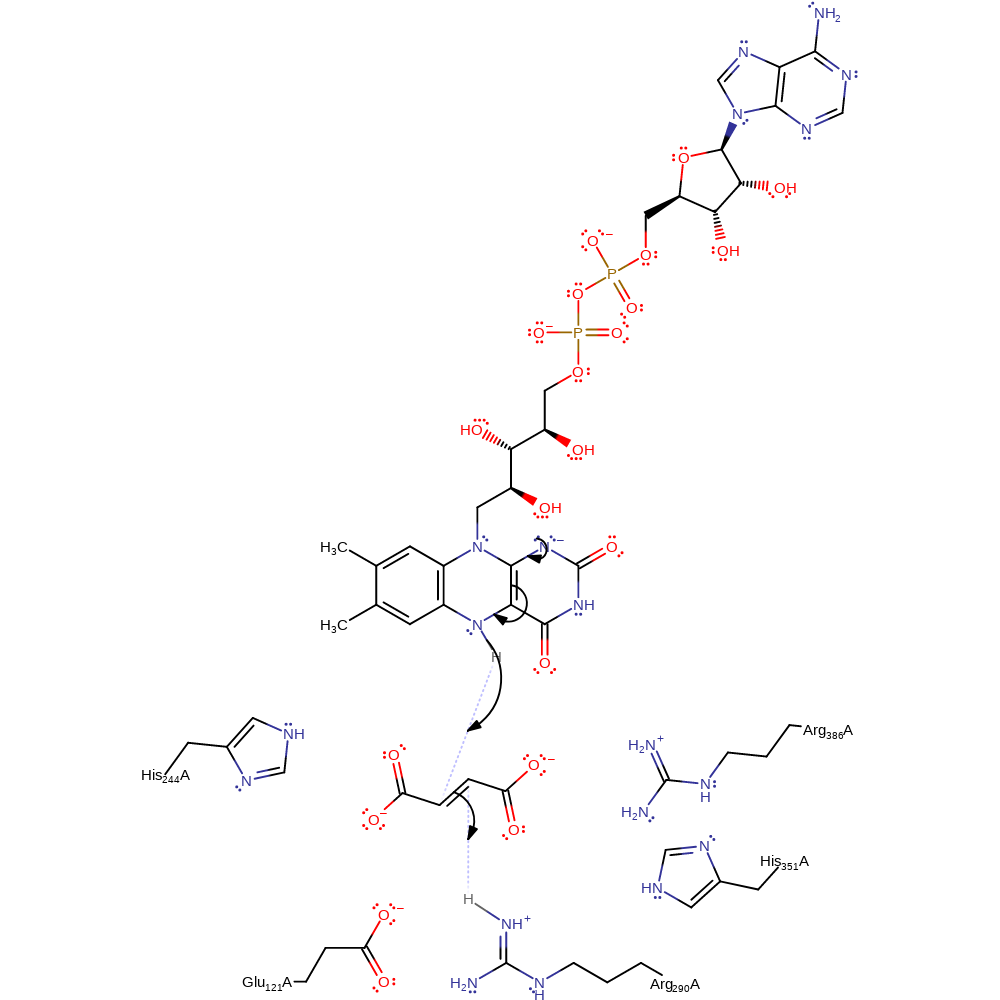
Step 2. FADH- is reoxidised by fumarate. A hydride is transferred from N5 of FADH- while fumarate accepts a proton from a general acid donor, most likely to be Arg290. Molecular oxygen can also be used to regenerate FAD from FADH2, forming hydrogen peroxide in the process.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu121A | electrostatic stabiliser |
| His244A | electrostatic stabiliser |
| His351A | electrostatic stabiliser |
| Arg290A | proton donor |


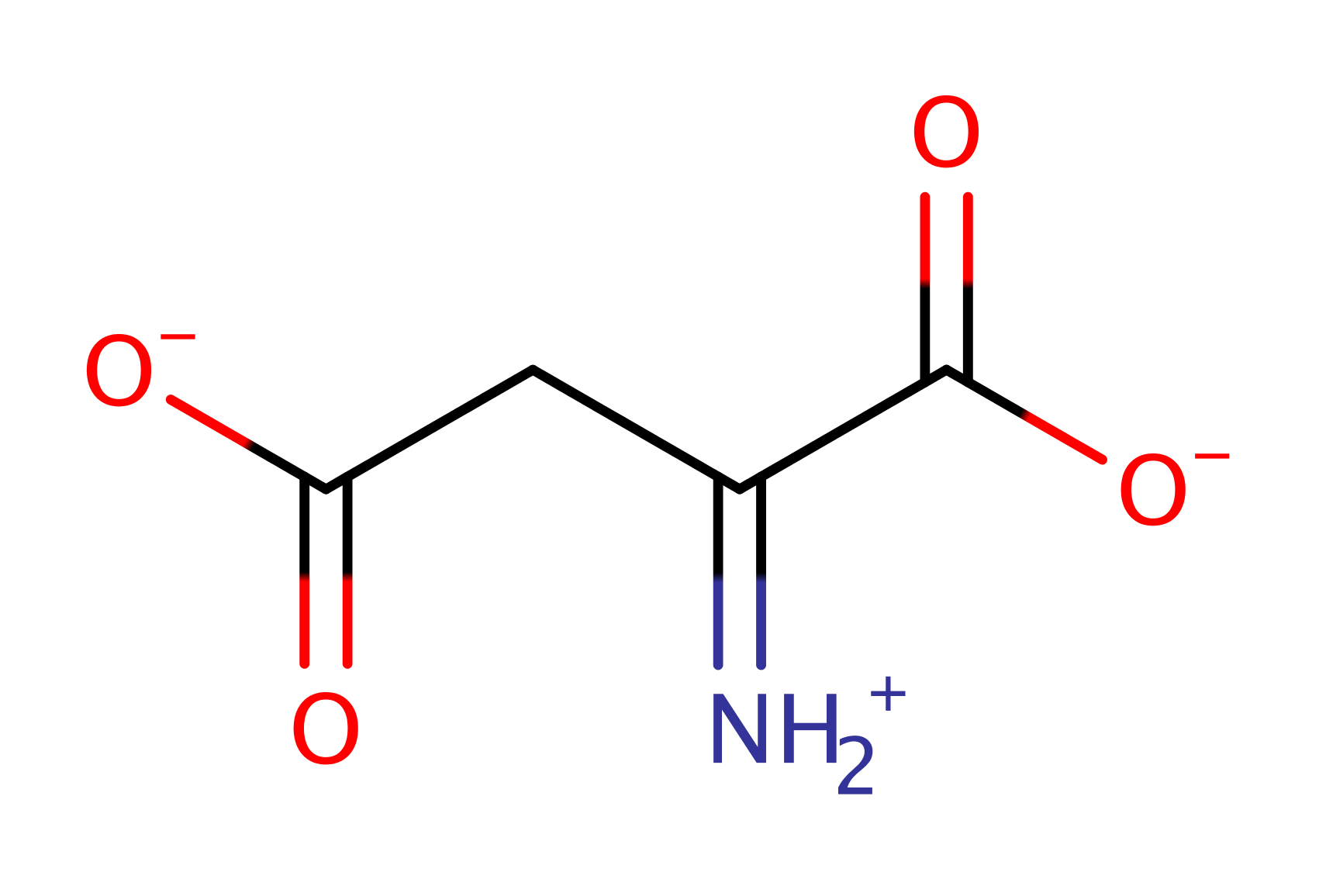

 Download:
Download:  Download:
Download: