Chloramphenicol O-acetyltransferase
Chloramphenicol acetyltransferase (CAT) [PMID:1867721] catalyses the acetyl-CoA dependent acetylation of chloramphenicol (Cm), an antibiotic which inhibits prokaryotic peptidyltransferase activity. Acetylation of Cm by CAT inactivates the antibiotic. A histidine residue, located in the C-terminal section of the enzyme, plays a central role in its catalytic mechanism. CAT enzymes are divided into three classes: CATI, CATII, and CATIII, with the enzymes of all three classes catalysing acetyl transfer to CAM to yield 3-O-acetyl-CAM. This entry represents the type III protein, although the mechanism is likely to be similar between the three classes.
Reference Protein and Structure
- Sequence
-
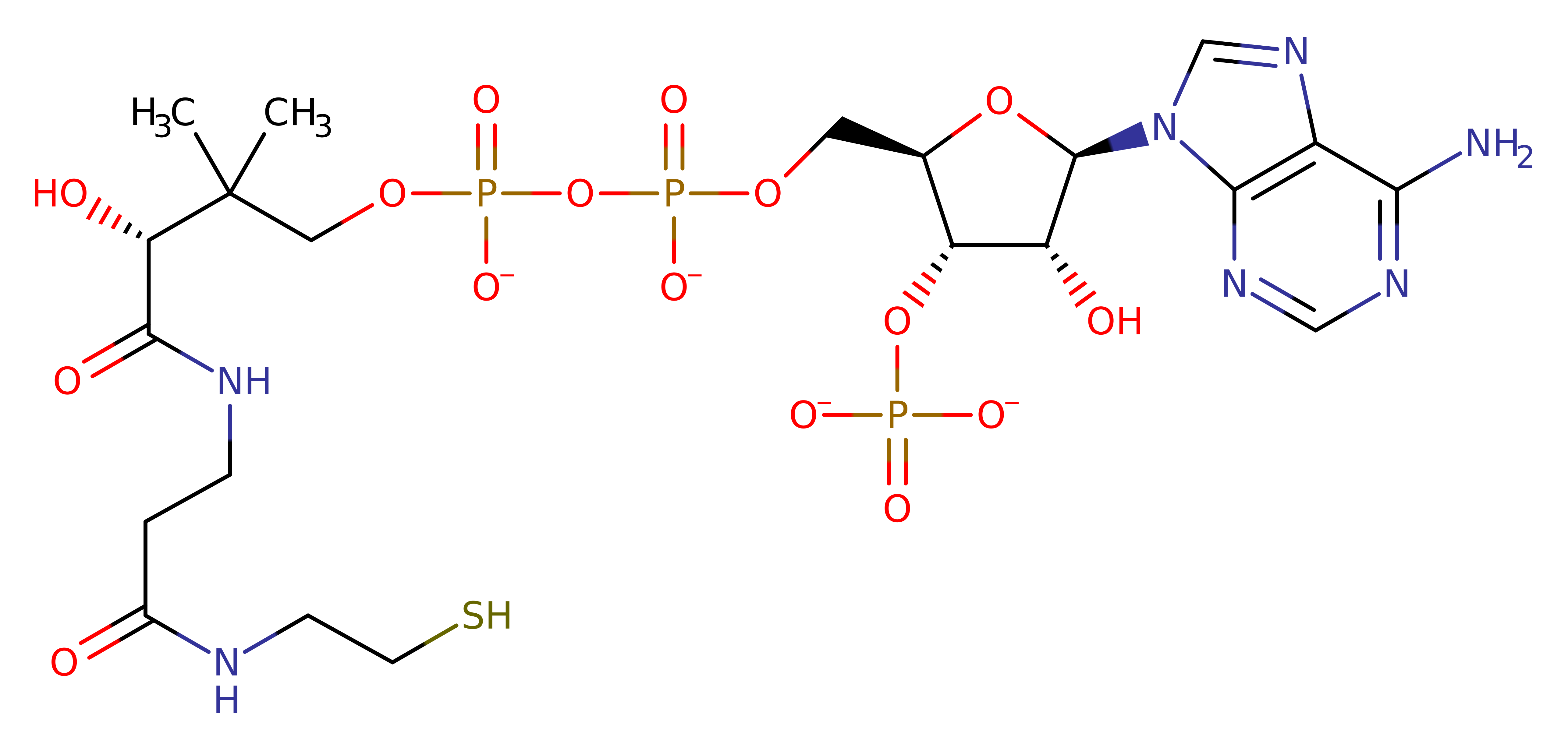
P00484
 (2.3.1.28)
(2.3.1.28)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Escherichia coli (Bacteria)

- PDB
-
3cla
- REFINED CRYSTAL STRUCTURE OF TYPE III CHLORAMPHENICOL ACETYLTRANSFERASE AT 1.75 ANGSTROMS RESOLUTION
(1.75 Å)



- Catalytic CATH Domains
-
3.30.559.10
 (see all for 3cla)
(see all for 3cla)
- Cofactors
- Water (1)
Enzyme Reaction (EC:2.3.1.28)
Enzyme Mechanism
Introduction
His195 abstracts the proton from the substrate hydroxyl group, which initiates a nucleophilic attack on the carbonyl carbon of the acyl CoA molecule. The oxyanion formed is stabilises by a hydrogen bonding network with a conserved water molecule and Thr174. The intermediate then collapses, forming the final products.
Catalytic Residues Roles
| UniProt | PDB* (3cla) | ||
| His189 | His195(189)A | His195 is appropriately positioned to act as a general base catalyst in the reaction, and the required tautomeric stabilisation is provided by an unusual interaction with a main-chain carbonyl oxygen [PMID: 2187098]. | proton shuttle (general acid/base) |
| Arg13, Asp193 | Arg18(13)A, Asp199(193)A | Form part of the Arg-Asp-His catalytic triad that activates the histidine to act as a general acid/base. | electrostatic stabiliser |
| Thr168 | Thr174(168)A(AB) | Helps stabilise the transition states formed during the course of the reaction. Thr174 bonds to water, which in turn forms a hydrogen bond to the transition state. | activator, transition state stabiliser |
Chemical Components
References
- Shaw WV et al. (1991), Annu Rev Biophys Biophys Chem, 20, 363-386. Chloramphenicol Acetyltransferase. DOI:10.1146/annurev.bb.20.060191.002051. PMID:1867721.
- Beaman TW et al. (1998), Biochemistry, 37, 6689-6696. Structure of the hexapeptide xenobiotic acetyltransferase from Pseudomonas aeruginosa. DOI:10.1021/bi980106v. PMID:9578552.
- Lewendon A et al. (1994), Biochemistry, 33, 1944-1950. Replacement of catalytic histidine-195 of chloramphenicol acetyltransferase: Evidence for a general base role for glutamate. DOI:10.1021/bi00173a043. PMID:7906544.
- Lewendon A et al. (1993), J Biol Chem, 268, 20997-21001. Transition state stabilization by chloramphenicol acetyltransferase. Role of a water molecule bound to threonine 174. PMID:8407936.
- Leslie AG (1990), J Mol Biol, 213, 167-186. Refined crystal structure of type III chloramphenicol acetyltransferase at 1·75 Å resolution. DOI:10.1016/s0022-2836(05)80129-9. PMID:2187098.
Catalytic Residues Roles
| Residue | Roles |
|---|---|
| His195(189)A | proton shuttle (general acid/base) |
| Thr174(168)A(AB) | activator, transition state stabiliser |
| Arg18(13)A | electrostatic stabiliser |
| Asp199(193)A | electrostatic stabiliser, modifies pKa |